Gas-Vapor Mixture Temperature in the Near-Surface Layer of a Rapidly-Evaporating Water Droplet
Abstract
:1. Introduction
2. Experimental Setup and Procedure
2.1. Heated Air Flow Parameters
2.2. Water Droplet
2.3. Plotting Temperature Fields Using LIP
2.4. Plotting Temperature Fields Using PLIF
2.5. Plotting Velocity Fields of Gas-Vapor Mixture Using PIV
2.6. Calculated Parameters of Processes under Study
3. Results and discussion
3.1. Unsteady Temperature Fields of an Evaporating Droplet and Gas-Vapor Mixture around It
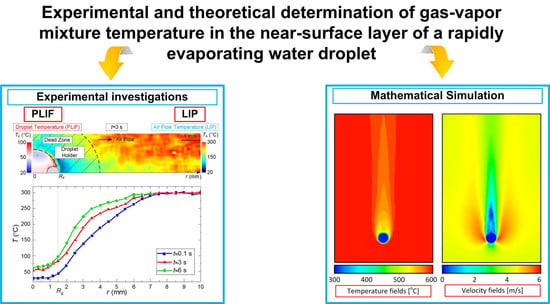
- First, the limitations of LIP in the recording of the temperature of the gas-vapor mixture around the droplet (Figure 5) have been established and viability of using the model from [22] is shown to correct the gas-vapor mixture temperature around the water droplet surface (i.e., in the dead zones of LIP). In general, applying interpolation and extrapolation methods, we can use the experimental points obtained with LIP in the gas-vapor area and with PLIF in the droplet to predict the temperatures in the gas-vapor layer around the droplet. Figure 5a,b shows that the temperature profiles in the experiments and when modeling are also in good agreement for the areas where it is impossible to plot reliable experimental fields; the prediction can be made using temperature distribution obtained by means of the model. These procedures were used when plotting such temperature distributions in Figure 6 with experimental values Td and Tg. Figure 7 shows that the absolute maximum temperature values in reference points coincide.
- Second, we clearly show the unsteady changes of temperature fields of heated and rapidly-evaporating water droplets (Figure 5 and Figure 7). A similar conclusion was made from the experimental results in study [31]. This factor is extremely important and determines the conditions and characteristics of water droplet heating when energy is supplied and spent on the phase transition. Traditionally, assumptions are made [12,13,14,15,16] that all the energy supplied to the water droplet surface is spent on the phase transition, and the process of its heating is not decisive. However, the temperature fields obtained illustrate that a water droplet has a highly inhomogeneous temperature field for a long time (temperature variations can be 70 to 80 °C). Therefore, it is important to take this factor into consideration in the mathematical modeling. The temperature fields, obtained when combining the results of processing using LIP and PLIF, show that the supplied energy is spent not only on the phase transition but also on heating the water droplet and the gas-vapor mixture. The heating of the gas-vapor mixture is a complex process: on the one hand, the energy of the air flow is spent on the heating of relatively cold vapors injected from the droplet surface, and on the other, the water vapors are known to contribute to additional radiation. Hence, in actual operating conditions, the vapors forming in the near-surface layer of an evaporating droplet strengthen the radiative heat exchange.
- Third, combining the temperature fields of PLIF and LIP brings about difficulties in the adequate control of temperature in the thin near-surface layer (Figure 5), as we plotted the temperature fields of the gas-vapor mixture and droplet within several pixels for the media interface. Therefore, it was reasonable (Figure 5 and Figure 6) to use an interpolation approach in this small-size region (i.e., plot temperature and temperature distribution fields for the dead zones of PLIF and LIP using the values in the extreme points of reliable regions in the liquid and vapor phases).
3.2. Generalized Curves
4. Conclusions
- Using the experimental results obtained by the optical techniques of PLIF and LIP, we have confirmed the hypotheses previously formulated by the authors of the manuscript (discussed in papers [22,23,24,31]) about a highly unsteady and inhomogeneous temperature field of a rapidly-evaporating water droplet, as well as a gas-vapor mixture around it and behind it (i.e., in its trace) with the incoming high-temperature gas flow.
- Using PLIF, we established that the temperature field of a droplet becomes quasi-steady only after 10%–15% of its complete evaporation time. The temperature trace of an evaporating droplet (i.e., a gas-vapor cloud) remains inhomogeneous due to a decrease in the droplet dimensions during evaporation, as well as in the convective mixing of cold water vapors and heated combustion products. By analyzing two main mechanisms of decreasing the temperature of the gas-vapor mixture in the trace of a water droplet, we established that the higher the temperature of the incoming gas flow, the more significantly it falls in the trace of an evaporating droplet. The endothermic phase transition plays a major role. Due to the convective heat exchange, it is difficult to considerably decrease the temperature in the droplet trace.
- A new approach has been proposed to the description of temperature fields in a system with rapidly-evaporating droplets of liquid. It is based on the experimental recording of temperature in PLIF and LIP availability zones with subsequent mathematical modeling of temperature fields of a small region inaccessible for these techniques. When modeling the corresponding problems of heat transfer in the boundary conditions, we used the temperature values determined in the experiments. The research findings are a backbone for the development of modern models of vaporization in the region of high temperatures with due consideration of the decisive influence of the thermal (vapor) insulation layer of an evaporating water droplet. Of particular value are the established consistent patterns of the processes of the buffer vapor layer formation around an evaporating droplet.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Cv | water vapor concentration, %; |
| htt | width of temperature trace of water droplet, mm; |
| hvtt | visible width of temperature trace of water droplet, mm; |
| hvt | width of aerodynamic (velocity) trace of water droplet, mm; |
| ltt | length of temperature trace of water droplet, mm; |
| lvt | length of aerodynamics (velocity) trace of water droplet, mm; |
| r | radial coordinate, mm; |
| Rd | initial droplet radius, mm; |
| T | temperature, °C; |
| Ta | air temperature, °C; |
| Td | temperature inside of water droplet, °C; |
| Ts | temperature of droplet surface, °C; |
| TSt.Dev | standard deviation of LIP method, °C; |
| Tt | temperature of vapor-air mixture in the trace of water droplet, °C; |
| ΔT | temperature drop in the droplet trace as compared to incoming air flow temperature (Ta–Tt), °C; |
| t | time, s; |
| Ua | velocity of air flow, m/s; |
| Ut | velocity of vapor-air mixture in the trace of water droplet, m/s; |
| ΔU | parameter for air velocity drop in the droplet trace as compared to incoming air flow velocity (Ua–Ut), m/s; |
| Vd | initial volume of water droplet, μL; |
| y | coordinate, mm; |
| νa | kinematic viscosity of air, m2/s; |
| Rea | Reynolds number for air. |
References
- Chiu, H.H. Advances and challenges in droplet and spray combustion. 1. Towards a unified theory of droplet and spray combustion. Prog. Energy Combust. Sci. 2000, 26, 381–416. [Google Scholar] [CrossRef]
- Sazhin, S.S.; Krutitskii, P.A.; Abdelghaffar, W.A.; Sazhina, E.M.; Mikhalovsky, S.V.; Meikle, S.T.; Heikal, M.R. Transient heating of diesel fuel droplets. Int. J. Heat Mass Transfer 2004, 47, 3327–3340. [Google Scholar] [CrossRef]
- Sazhin, S.S. Advanced models of fuel droplet heating and evaporation. Prog. Energy Combust. Sci. 2006, 32, 162–214. [Google Scholar] [CrossRef]
- Jerman, I.; Ratajc, P. A Further indication of the self-ordering capacity of water via the droplet evaporation method. Entropy 2014, 16, 5211–5222. [Google Scholar] [CrossRef]
- Sazhin, S.S. Modelling of fuel droplet heating and evaporation: Recent results and unsolved problems. Fuel 2017, 196, 69–101. [Google Scholar] [CrossRef]
- Sazhin, S.S.; Abdelghaffar, W.A.; Sazhina, E.M.; Heikal, M.R. Models for droplet transient heating: effects on droplet evaporation, ignition, and break-up. Int. J. Therm. Sci. 2005, 44, 610–622. [Google Scholar] [CrossRef]
- Sawant, P.; Ishii, M.; Mori, M. Prediction of amount of entrained droplets in vertical annular two-phase flow. Int. J. Heat Fluid Flow 2009, 30, 715–728. [Google Scholar] [CrossRef]
- Croquer, S.; Poncet, S.; Aidoun, Z. Thermodynamic modelling of supersonic gas ejector with droplets. Entropy 2017, 19, 579. [Google Scholar] [CrossRef]
- Kwak, H.-Y. Entropy generation due to the heat transfer for evolving spherical objects. Entropy 2018, 20, 562. [Google Scholar] [CrossRef]
- Brin, A.A.; Fisenko, S.P.; Khodyko, Y.A. Characteristic features of evaporative cooling of droplets in high-temperature flows. J. Eng. Phys. Thermophys. 2011, 84, 292–297. [Google Scholar] [CrossRef]
- Harstad, K.G.; Le Clercq, P.C.; Bellan, J. Statistical model of multicomponent-fuel drop evaporation for many-drop flow simulations. AIAA J. 2003, 41, 1858–1874. [Google Scholar] [CrossRef]
- Zeng, Y.; Lee, C.-F. A preferential vaporization model for multicomponent droplets and sprays. Atomization Sprays 2002, 12, 163–186. [Google Scholar] [CrossRef]
- Terekhov, V.I.; Terekhov, V.V.; Shishkin, N.E.; Bi, K.C. Heat and mass transfer in disperse and porous media experimental and numerical investigations of nonstationary evaporation of liquid droplets. J. Eng. Phys. Thermophys. 2010, 83, 883–890. [Google Scholar] [CrossRef]
- Yagov, V.V.; Minko, M.V. Simulating droplet entrainment in adiabatic disperse-annular two-phase flows. Therm. Eng. 2013, 60, 65–70. [Google Scholar] [CrossRef]
- Yuen, M.C.; Chen, L.W. Heat-transfer measurements of evaporating liquid droplets. Int. J. Heat Mass Transfer 1978, 21, 537–542. [Google Scholar] [CrossRef]
- Renksizbulut, M.; Yuen, M.C. Numerical study of droplet evaporation in a high-temperature stream. J. Heat Transfer 1983, 105, 389–397. [Google Scholar] [CrossRef]
- Vysokomornaya, O.V.; Kuznetsov, G.V.; Strizhak, P.A. Evaporation of water droplets in a high-temperature gaseous medium. J. Eng. Phys. Thermophys. 2016, 89, 141–151. [Google Scholar] [CrossRef]
- Spalding, D.B. Some fundamentals of combustion; Butterworth Scientific: London, UK, 1955; pp. 1–250. [Google Scholar]
- Volkov, R.S.; Vysokomornaya, O.V.; Kuznetsov, G.V.; Strizhak, P.A. Experimental study of the change in the mass of water droplets in their motion through high-temperature combustion products. J. Eng. Phys. Thermophys. 2013, 86, 1413–1418. [Google Scholar] [CrossRef]
- Ranz, W.E.; Marshall, W.R. Evaporation from drops. Chem. Eng. Progr. 1952, 48, 141–146, 173–180. [Google Scholar]
- Kuznetsov, G.V.; Kuibin, P.A.; Strizhak, P.A. Estimation of the numerical values of the evaporation constants of the water drops moving in the high temperature gas flow. High Temp. 2015, 53, 254–258. [Google Scholar] [CrossRef]
- Antonov, D.V.; Kuznetsov, G.V.; Strizhak, P.A. Determination of temperature and concentration of a vapor–gas mixture in a wake of water droplets moving through combustion products. J. Eng. Thermophys. 2016, 25, 337–351. [Google Scholar] [CrossRef]
- Kuznetsov, G.V.; Strizhak, P.A.; Volkov, R.S. Temperature measurement in the trace of water droplet when heating by hot air. Exp. Therm. Fluid Sci. 2017, 81, 256–264. [Google Scholar] [CrossRef]
- Kuznetsov, G.V.; Strizhak, P.A.; Volkov, R.S.; Voytkov, I.S. Gas temperature in the trace of water droplets streamlined by hot air flow. Int. J. Multiphase Flow 2017, 91, 184–193. [Google Scholar] [CrossRef]
- Lavieille, P.; Lemoine, F.; Lavergne, G.; Virepinte, J.F.; Lebouche, M. Temperature measurements on droplets in monodisperse stream using laser-induced fluorescence. Exp. Fluids 2000, 29, 429–437. [Google Scholar]
- Castanet, G.; Labergue, A.; Lemoine, F. Internal temperature distributions of interacting and vaporizing droplets. Int. J. Therm. Sci. 2011, 50, 1181–1190. [Google Scholar] [CrossRef] [Green Version]
- Castanet, G.; Perrin, L.; Caballina, O.; Lemoine, F. Evaporation of closely-spaced interacting droplets arranged in a single row. Int. J. Heat Mass Transfer 2016, 93, 788–802. [Google Scholar] [CrossRef] [Green Version]
- Lemoine, F.; Castanet, G. Temperature and chemical composition of droplets by optical measurement techniques: A state-of-the-art review. Exp. Fluids. 2013, 54, 1572. [Google Scholar] [CrossRef]
- Akhmetbekov, Y.K.; Alekseenko, S.V.; Dulin, V.M.; Markovich, D.M.; Pervunin, K.S. Planar fluorescence for round bubble imaging and its application for the study of an axisymmetric two-phase jet. Exp. Fluids 2010, 48, 615–629. [Google Scholar] [CrossRef]
- Abram, C.; Fond, B.; Beyrau, F. Temperature measurement techniques for gas and liquid flows using thermographic phosphor tracer particles. Prog. Energy Combust. Sci. 2018, 64, 93–156. [Google Scholar] [CrossRef]
- Strizhak, P.A.; Volkov, R.S.; Castanet, G.; Lemoine, F.; Rybdylova, O.; Sazhin, S.S. Heating and evaporation of suspended water droplets: experimental studies and modelling. Int. J. Heat Mass Transfer 2018, 127, 92–106. [Google Scholar] [CrossRef]
- Volkov, R.S.; Strizhak, P.A. Planar laser-induced fluorescence diagnostics of water droplets heating and evaporation at high-temperature. Appl. Therm. Eng. 2017, 127, 141–156. [Google Scholar] [CrossRef]
- Volkov, R.S.; Kuznetsov, G.V.; Strizhak, P.A. Temperature and velocity fields of the gas-vapor flow near evaporating water droplets. Int. J. Therm. Sci. 2018, 134, 337–354. [Google Scholar] [CrossRef]
- Zaripov, T.S.; Rybdylova, O.; Sazhin, S.S. A model for heating and evaporation of a droplet cloud and its implementation into ANSYS Fluent. Int. Commun. Heat Mass Transfer 2018, 97, 85–91. [Google Scholar] [CrossRef]
- Korobeinichev, O.P.; Shmakov, A.G.; Shvartsberg, V.M.; Chernov, A.A.; Yakimov, S.A.; Koutsenogii, K.P.; Makarov, V.I. Fire suppression by low-volatile chemically active fire suppressants using aerosol technology. Fire Saf. J. 2012, 51, 102–109. [Google Scholar] [CrossRef]
- Misyura, S.Y. Contact angle and droplet heat transfer during evaporation on structured and smooth surfaces of heated wall. Appl. Surf. Sci. 2017, 414, 188–196. [Google Scholar] [CrossRef]
- Kichatov, B.; Korshunov, A.; Kiverin, A.; Son, E. Experimental study of foamed emulsion combustion: Influence of solid microparticles, glycerol and surfactant. Fuel Proces. Technol. 2017, 166, 77–85. [Google Scholar] [CrossRef]
- Zhang, Z.; Bei, H.; Li, H.; Li, X.; Gao, X. Understanding the co-pyrolysis behavior of indonesian oil sands and corn straw. Energy Fuels 2017, 31, 2538–2547. [Google Scholar] [CrossRef]
- Glazov, S.V.; Kislov, V.M.; Salgansky, E.A.; Rabinovich, O.S.; Malinouski, A.I.; Salganskaya, M.V.; Pilipenko, E.N.; Kolesnikova, Y.Y. Effect of local rearrangements in the particle bed on the stability of filtration combustion of solid fuel. Int. J. Heat Mass Transfer 2017, 108, 1602–1609. [Google Scholar] [CrossRef]
- Varaksin, A.Y. Fluid dynamics and thermal physics of two-phase flows: Problems and achievements. High Temp. 2013, 51, 377–407. [Google Scholar] [CrossRef]














© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonov, D.; Volkov, R.; Strizhak, P. Gas-Vapor Mixture Temperature in the Near-Surface Layer of a Rapidly-Evaporating Water Droplet. Entropy 2019, 21, 803. https://doi.org/10.3390/e21080803
Antonov D, Volkov R, Strizhak P. Gas-Vapor Mixture Temperature in the Near-Surface Layer of a Rapidly-Evaporating Water Droplet. Entropy. 2019; 21(8):803. https://doi.org/10.3390/e21080803
Chicago/Turabian StyleAntonov, Dmitry, Roman Volkov, and Pavel Strizhak. 2019. "Gas-Vapor Mixture Temperature in the Near-Surface Layer of a Rapidly-Evaporating Water Droplet" Entropy 21, no. 8: 803. https://doi.org/10.3390/e21080803
APA StyleAntonov, D., Volkov, R., & Strizhak, P. (2019). Gas-Vapor Mixture Temperature in the Near-Surface Layer of a Rapidly-Evaporating Water Droplet. Entropy, 21(8), 803. https://doi.org/10.3390/e21080803