Synthesis, Characterization and Antimicrobial Activity of New 1,2,3-Selenadiazoles
Abstract
:Introduction
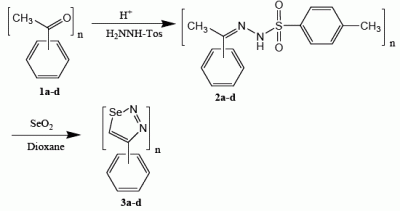
Results and Discussion


| Compound | Conc. (g/mL) | Pathogens | ||||
|---|---|---|---|---|---|---|
| Aa | B | C | D | E | ||
| Tobramycin | 0.010 | 15b | 13 | 22 | - | 14 |
| 0.005 | 13 | 11 | 17 | - | 8 | |
| 2a | 0.010 | 11 | 12 | 12 | - | 9 |
| 0.005 | 8 | 10 | 8 | - | - | |
| 2b | 0.010 | 13 | 13 | 14 | - | 8 |
| 0.005 | 8 | 8 | 9 | - | - | |
| 2c | 0.010 | - | 8 | 11 | - | - |
| 0.005 | - | - | 8 | - | - | |
| 2d | 0.010 | - | 9 | 10 | - | 8 |
| 0.005 | - | - | 8 | - | - | |
| 3a | 0.010 | 8 | 12 | 10 | 8 | 14 |
| 0.005 | - | 9 | 8 | - | 10 | |
| 3b | 0.010 | 9 | 11 | 11 | - | 15 |
| 0.005 | - | 10 | 8 | - | 12 | |
| 3c | 0.010 | 8 | 8 | 10 | 8 | 14 |
| 0.005 | - | - | 9 | - | 10 | |
| 3d | 0.010 | - | - | 8 | - | 8 |
| 0.005 | - | - | - | - | - | |
| 3e | 0.010 | 13 | 13 | 19 | 10 | 15 |
| 0.005 | 13 | - | 14 | 9 | 9 | |
| Compound | Conc. (g/mL) | Pathogens | ||||
|---|---|---|---|---|---|---|
| Aa | B | C | D | E | ||
| Tobramycin | 0.010 | 16b | 14 | 24 | - | 16 |
| 0.005 | 10 | 12 | 16 | - | 10 | |
| 2a | 0.010 | 10 | 13 | 14 | - | 9 |
| 0.005 | 8 | 10 | 8 | - | - | |
| 2b | 0.010 | 13 | 14 | 15 | - | 8 |
| 0.005 | 9 | 10 | 9 | - | - | |
| 2c | 0.010 | - | 9 | 13 | - | 9 |
| 0.005 | - | - | 9 | - | - | |
| 2d | 0.010 | 8 | 9 | 9 | - | 9 |
| 0.005 | - | - | - | - | - | |
| 3a | 0.010 | 8 | 13 | 14 | 9 | 15 |
| 0.005 | - | 10 | 8 | - | 11 | |
| 3b | 0.010 | 10 | 11 | 12 | - | 16 |
| 0.005 | - | 10 | 9 | - | 11 | |
| 3c | 0.010 | 8 | 8 | 11 | 8 | 15 |
| 0.005 | - | - | 10 | - | 11 | |
| 3d | 0.010 | 8 | - | 9 | - | 9 |
| 0.005 | - | - | - | - | - | |
| 3e | 0.010 | 15 | 14 | 21 | 13 | 18 |
| 0.005 | 14 | 10 | 15 | 10 | 11 | |
Experimental
General
General procedure for the preparation of the tosylhydrazones 2a-d
General procedure for the preparation of the 1,2,3-selenadiazole derivatives 3a-d [16, 22]
3-[4-(1,2,3-Selenadiazole-4-yl)phenoxy]-1,2-propenoxide (3e)
Microbiology
Acknowledgements
References
- Bakulev, V. A.; Dehaen, W. The Chemistry of 1,2,3-Thiadiazoles; John Wiley & Sons, Inc: New York, NY, USA, 2004; pp. 193–228. [Google Scholar]
- Gleiter, R.; Schehlmann, V. Formation of noval strained cycloalkynes from fused 1,2,3-selena-diazoles. Angew. Chem. 1990, 102, 1450–1456. [Google Scholar] [CrossRef]
- Kandeel, M.; El-meligie, S.; Omar, R.; Roshdy, S.; Youssef, K. Synthesis of certain 1,2,3-selena-diazole, 1,2,3-thiadiazole and 1,2-oxazoline derivatives of anticipated antibacterial activity. J. Pharm. Sci. 1994, 3, 197–205. [Google Scholar]
- Lalezari, I.; Shafiee, A.; Yazdany, S. Selenium heterocycles. X. Synthesis and Antibacterial activity of pyridyl-1,2,3-thiadiazoles and pyridyl-1,2,3-selenadiazoles. J. Pharm. Sci. 1974, 63, 628–629. [Google Scholar] [CrossRef]
- El-Bahaie, S.; Assy, M. G.; Hassanien, M. M. Synthesis and antibacterial activity of 1,2,3-selenadiazoles. J. Indian Chem. Soc. 1990, 67, 757–763. [Google Scholar]
- Lalezari, I.; Shafiee, A.; Khorrami, J.; Soltani, A. Synthesis and antibacterial and antifungal activities of Arylsulfonyl-1,2,3-selenadiazoles. J. Pharm. Sci. 1978, 67, 1336–1338. [Google Scholar] [CrossRef]
- Jailian, A. R.; Sattari, S.; Bineshmarvasti, M.; Daneshtalab, M.; Shafiee, A. Synthesis and in vitro antifungal and cytotoxicity evaluation of substituted 4,5-dihydronaphtho[1,2-d][1,2,3]thia (or selena)diazoles. Farmaco 2003, 58, 63–68. [Google Scholar] [CrossRef]
- Moawad, E. B.; Yousif, M. Y.; Metwally, M. A. Synthesis of certain heteroaryl-fused pyrimidines and pyridines and selena and thiadiazoles with naphthyl substituent as potential antifungal agents. Pharmazie 1989, 44, 820–822. [Google Scholar]
- Reddy, D. B.; Reddy, A. S.; Sekhar, T. C.; Padmavathi, V. Study of the biological activity of 4-(3-pyridyl)-5-phenylsulfonyl-1,2,3-selenadiazole and derivatives. J. Ecotoxicol. Environ. Monitor 1999, 3-4, 225–229. [Google Scholar]
- Pawar, R. B.; Muwad, V. V. Synthesis of some biologically active pyrazole, thiazolidinone and azetidinone derivatives. J. Ecotoxicol. Environ. Monit. 1999, 3-4, 225–229. [Google Scholar]
- Katrizky, A. R.; Rees, C. W. (Eds.) Comprehensive Heterocyclic Chemistry; Pergamon Press: Oxford, U. K, 1984; Vol. 4, Chapters 1-4 and Vol. 5, Chapters 1-3.
- Baht, B. A.; Dhar, K. L.; Puri, S. C.; Saxena, A. K. I. Synthesis and biological evaluation of chalcones and their derived pyrazoles as potential cytotoxic. Bioorg. Med. Chem. Lett. 2005, 15, 3177–3180. [Google Scholar] [CrossRef]
- Arsenyan, P.; Rubina, K.; Shestakova, I.; Domracheva, I. 4-Methyl-1,2,3-selenadiazole-5-carboxylic acid amids: antitumor action and cytotoxic effect correlation. Eur. J. Med. Chem. 2007, 42, 635–40. [Google Scholar] [CrossRef]
- Chen, T.; Zheng, W.; Wong, Y. S.; Yang, F. Mitochondria-mediated apoptosis in human breast carcinoma MCF-7 cells induced by a novel selenadiazole derivative. Biomed. Pharmacother. 2008, 62, 77–84. [Google Scholar] [CrossRef]
- Klayman, D. L.; Günther, W. H. H. Organic Selenium Compounds: Their Chemistry and Biology; John Wiley & Sons, Inc.: New York, NY, USA, 1973; pp. 464–491. [Google Scholar]
- Al-Smadi, M.; Ratrout, S. New 1,2,3-Selenadiazole and 1,2,3-Thiadiazole derivatives. Molecules 2004, 9, 957–967. [Google Scholar] [CrossRef]
- Al-Smadi, M.; Meier, H. Multi-arm 1,2,3-Thiadiazole systems. Liebigs Ann. 1997, 2357–2361. [Google Scholar] [CrossRef]
- Al-Smadi, M.; Hanold, N.; Meier, H. Multiple 1,2,3-thiadiazoles. J. Heterocycl. Chem. 1997, 34, 605–611. [Google Scholar] [CrossRef]
- Lalezari, I.; Shafiee, A. A noval synthesis of selenium heterocycles: substituted 1,2,3-Selenadiazoles. Tetrahedron Letters 1969, 58, 5105–5106. [Google Scholar] [CrossRef]
- Golgolab, H.; Lalezari, I. Selenium Heterocycles. XVI. 1,2,3-selenadiazole derivatives of polycyclic aromatic compounds and steroids (1). J. Heterocycl. Chem. 2002, 41, 2489–2493. [Google Scholar]
- Banty, A. L. The Antimicrobial Susceptibility Test; Principles and Practice; Lea and Febiger: Philadelphia, PA, USA, 1979; pp. 180–212. [Google Scholar]
- Seeley, H.W.; Van Demark, P. J. Microbes in action: Alaboratory manual of Microbiology; D. B. Taraporewala Sons and Co Pvt Ltd: Mumbai, India, 1975; pp. 55–83. [Google Scholar]
- Sample availability: Available from the authors.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Al-Smadi, M.; Al-Momani, F. Synthesis, Characterization and Antimicrobial Activity of New 1,2,3-Selenadiazoles. Molecules 2008, 13, 2740-2749. https://doi.org/10.3390/molecules13112740
Al-Smadi M, Al-Momani F. Synthesis, Characterization and Antimicrobial Activity of New 1,2,3-Selenadiazoles. Molecules. 2008; 13(11):2740-2749. https://doi.org/10.3390/molecules13112740
Chicago/Turabian StyleAl-Smadi, Mousa, and Fouad Al-Momani. 2008. "Synthesis, Characterization and Antimicrobial Activity of New 1,2,3-Selenadiazoles" Molecules 13, no. 11: 2740-2749. https://doi.org/10.3390/molecules13112740
APA StyleAl-Smadi, M., & Al-Momani, F. (2008). Synthesis, Characterization and Antimicrobial Activity of New 1,2,3-Selenadiazoles. Molecules, 13(11), 2740-2749. https://doi.org/10.3390/molecules13112740