A New Phloroglucinol Diglycoside Derivative from Hypericum japonicum Thunb.
Abstract
:Introduction
Results and Discussion
 -24.5°(c. 0.5, MeOH), m.p. 220~223°C. Its molecular formula was deduced as C25H38O13 from the quasi-molecular ion peak at 569.2200 ([M + Na]+, calcd. 569.2260) in HR-ESI-MS spectrum. The IR spectrum exhibited the absorptions at 3350 cm-1 (OH), 1630 cm-1 (carbonyl), 1598 cm-1 and 1586 cm-1 (phenyl). Its 1H-NMR spectrum (Table 1) showed one hydroxyl proton at δ 12.48 (1H, br s). It also showed five methyl groups at δ 2.10 (3H, s), 1.97 (3H, s), 1.07 (3H, d, J = 6.1 Hz), 0.91 (3H, d, J = 7.2 Hz) and 0.87 (3H, t, J =7.5 Hz), and two anomeric protons at δ 4.42 (1H, br s) and 4.29 (1H, d, J = 7.7 Hz). The 13C-NMR spectrum showed six aromatic signals (δ 160.0, 158.3, 152.7, 110.1, 109.9, 107.5) and one carbonyl signal (δ 210.0). Combining with DEPT and 1H-NMR data, we deduced that the benzene ring was completely substituted. Signals at δ 3.0~3.5 in the 1H-NMR spectrum and signals at δ 60~80 in the 13C-NMR spectrum suggested two sugar units.
-24.5°(c. 0.5, MeOH), m.p. 220~223°C. Its molecular formula was deduced as C25H38O13 from the quasi-molecular ion peak at 569.2200 ([M + Na]+, calcd. 569.2260) in HR-ESI-MS spectrum. The IR spectrum exhibited the absorptions at 3350 cm-1 (OH), 1630 cm-1 (carbonyl), 1598 cm-1 and 1586 cm-1 (phenyl). Its 1H-NMR spectrum (Table 1) showed one hydroxyl proton at δ 12.48 (1H, br s). It also showed five methyl groups at δ 2.10 (3H, s), 1.97 (3H, s), 1.07 (3H, d, J = 6.1 Hz), 0.91 (3H, d, J = 7.2 Hz) and 0.87 (3H, t, J =7.5 Hz), and two anomeric protons at δ 4.42 (1H, br s) and 4.29 (1H, d, J = 7.7 Hz). The 13C-NMR spectrum showed six aromatic signals (δ 160.0, 158.3, 152.7, 110.1, 109.9, 107.5) and one carbonyl signal (δ 210.0). Combining with DEPT and 1H-NMR data, we deduced that the benzene ring was completely substituted. Signals at δ 3.0~3.5 in the 1H-NMR spectrum and signals at δ 60~80 in the 13C-NMR spectrum suggested two sugar units.
 of rhamnose is -4.4°, the
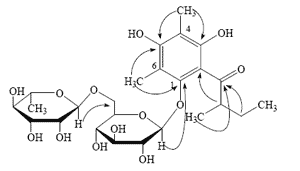
of rhamnose is -4.4°, the  of glucose is +52.5°), the configuration of the rhamnose was identified as α-L, while that of the glucose was β-D. Thus, compound 1 was identified as 4,6-dimethyl-1-O-[α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranosyl] multifidol. The complete assignments of 1H- and 13C-NMR data were based on the analyses of HSQC, 1H-1H COSY, and HMBC spectra (Figure 1). The key 1H-13C long-range correlations could be observed from H-1 of the rhamnose unit at δ 4.42 to C-6 of the glucopyranose unit at δ 67.4, from H-1 of the glucopyranose unit at δ 4.29 to C-1 of the phenyl unit at ä 152.7, and from H-2 of the methylbutyryl chain at δ 3.88 to C-2 of the phenyl unit at δ 110.1 in the HMBC spectrum.
of glucose is +52.5°), the configuration of the rhamnose was identified as α-L, while that of the glucose was β-D. Thus, compound 1 was identified as 4,6-dimethyl-1-O-[α-L-rhamnopyranosyl-(1→6)-β-D-glucopyranosyl] multifidol. The complete assignments of 1H- and 13C-NMR data were based on the analyses of HSQC, 1H-1H COSY, and HMBC spectra (Figure 1). The key 1H-13C long-range correlations could be observed from H-1 of the rhamnose unit at δ 4.42 to C-6 of the glucopyranose unit at δ 67.4, from H-1 of the glucopyranose unit at δ 4.29 to C-1 of the phenyl unit at ä 152.7, and from H-2 of the methylbutyryl chain at δ 3.88 to C-2 of the phenyl unit at δ 110.1 in the HMBC spectrum.| Position | δH | δC | Position | δH | δC |
|---|---|---|---|---|---|
| 1 | - | 210.0 | 1 | 4.29 (1H, d, 7.7) | 104.0 |
| 2 | 3.88 (1H, m) | 44.9 | 2 | 3.28 (1H, m) | 74.1 |
| 2-CH3 | 0.91 (3H, d, 7.2) | 17.9 | 3 | 3.19 (1H, m) | 76.2 |
| 3 | 1.31 (1H, m) 1.80 (1H, m) | 24.6 | 4 | 3.30 (1H, m) | 70.7 |
| 4 | 0.87 (3H, t, 7.5) | 11.9 | 5 | 3.10 (1H, m) | 75.0 |
| Phenyl unit | 6 | 3.69 (2H, d, 10.5) | 67.4 | ||
| 1 | - | 152.7 | Rhamnose unit | ||
| 2 | - | 110.1 | 1 | 4.42 (1H, br s) | 101.0 |
| 3 | - | 158.3 | 2 | 3.50 (1H, m) | 70.1 |
| 4 | - | 107.5 | 3 | 3.29 (1H, m) | 70.2 |
| 4-CH3 | 1.97 (3H, s) | 8.6 | 4 | 3.13 (1H, m) | 72.0 |
| 5 | - | 160.0 | 5 | 3.27 (1H, m) | 68.3 |
| 6 | - | 109.9 | 6 | 1.07 (3H, d, 6.1) | 17.8 |
| 6-CH3 | 2.10 (3H, s) | 9.5 | |||

Antihypoxic activity of Hypericum japonicum Thunb extracts
| Substances | Dose | Mice number (n) | Tolerance time (min) |
|---|---|---|---|
| DMSO diluted solution | 0.5 mL | 20 | 33.30 ± 7.23 |
| 60% EtOH extract | 20 mg/0.5 mL | 10 | 38.47 ± 9.22 |
| CHCl3 extract | 20 mg/0.5 mL | 10 | 40.15 ± 9.58 |
| EtOAc extract | 20 mg/0.5 mL | 10 | 44.37 ± 10.25 |
| n-BuOH extract | 20 mg/0.5 mL | 10 | 38.37± 8.18 |
| Water extract | 20 mg/0.5 mL | 10 | 33.62 ± 11.30 |
Conclusions
Experimental
General
Plant material
Extraction
Acknowledgements
References
- Grand Dictionary of Chinese Traditional Medicine; (Book One); Shanghai Science & Technology Press: Shanghai, P.R. China, 1977; pp. 813–814.
- Kosasi, S.; van der Sluis, W. G.; Labadie, R. P. Multifidol and multifidol glucoside from the latex of Jatropha multifida. Photochemistry 1989, 28, 2439–2441. [Google Scholar] [CrossRef]
- Wu, Q. L.; Wang, S. P.; Wang, L. W.; Yang, J. S.; Xiao, P. G. New phloroglucinol glycosides from Hypericum japonicum. Chin. Chem. Lett. 1998, 9, 469–470. [Google Scholar]
- Gao, W.; Shen, Y.; Zhang, H. J.; Tang, H.; Lin, H. W.; Qiu, F. The chemical constituents of Potentilla chinensis. Pharm. Care Res. 2007, 7, 262–264. [Google Scholar]
- Ma, X. F.; Tian, W. X.; Wu, L. H.; Cao, X. L.; Ito, Y. C. Isolation of quercetin-3-O-L-rhamnoside from Acer truncatum Bunge by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1070, 211–214. [Google Scholar] [CrossRef]
- Zhang, L.; Jin, Y. Y.; Tian, J. K. Studies on Chemical Constituents of Hypericum japonicum. Chin. Pharm. J. 2007, 42, 341–344. [Google Scholar]
- Agnihotri, V. K.; ElSohly, H. N.; Khan, S. I.; Smillie, T. J.; Khan, I. A.; Walker, L. A. Antioxidant constituents of Nymphaea caerulea flowers. Phytochemistry 2008, 69, 2061–2066. [Google Scholar] [CrossRef]
- Ding, L. S.; Liang, Q. L.; Teng, Y. F. Study on flavonoids in seeds of Hovenia dulcis. Acta Pharm. Sin. 1997, 32, 600–602. [Google Scholar]
- Shilova, I. V.; Pisareva, S. I.; Krasnov1, E. A.; Bruzhes1, M. A.; Pyak, A. I. Antioxidant properties of Bergenia crassifolia extract. Pharm. Chem. J. 2006, 40, 620–623. [Google Scholar] [CrossRef]
- Fu, F.; Li, T. Z.; Liu, R. H.; Zhang, W.; Zhang, C.; Zhang, W. D.; Chen, H. S. Studies on the Flavonoids of Hypericum japonicum Thunb. ex Murray. Chin. J. Nat. Med. 2004, 2, 283–284. [Google Scholar]
- Teng, R. W.; Zhou, Z. H.; Wang, D. Z.; Yang, C. T. Four Caffeoylquinic Acids from Morina Nepalensis Var. Alba Hand.-Mazz. Chin. J. Magn. Reson. 2002, 9, 167–174. [Google Scholar]
- Sample Availability: Contact the authors.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, X.W.; Mao, Y.; Wang, N.-L.; Yao, X.S. A New Phloroglucinol Diglycoside Derivative from Hypericum japonicum Thunb. Molecules 2008, 13, 2796-2803. https://doi.org/10.3390/molecules13112796
Wang XW, Mao Y, Wang N-L, Yao XS. A New Phloroglucinol Diglycoside Derivative from Hypericum japonicum Thunb. Molecules. 2008; 13(11):2796-2803. https://doi.org/10.3390/molecules13112796
Chicago/Turabian StyleWang, Xiao Wei, Yu Mao, Nai-Li Wang, and Xin Sheng Yao. 2008. "A New Phloroglucinol Diglycoside Derivative from Hypericum japonicum Thunb." Molecules 13, no. 11: 2796-2803. https://doi.org/10.3390/molecules13112796
APA StyleWang, X. W., Mao, Y., Wang, N. -L., & Yao, X. S. (2008). A New Phloroglucinol Diglycoside Derivative from Hypericum japonicum Thunb. Molecules, 13(11), 2796-2803. https://doi.org/10.3390/molecules13112796