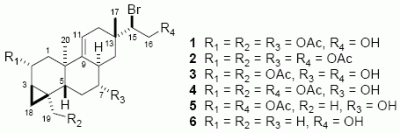Halogenated Terpenes and a C15-Acetogenin from the Marine Red Alga Laurencia saitoi
Abstract
:Introduction
Results and Discussion

| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 37.4 t | 37.4 t | 37.5 t | 37.4 t | 38.2 t | 31.1 t | 30.3 t | 31.0 q | 31.0 q | 73.9 d |
| 2 | 68.2 d | 68.2 d | 68.3 d | 68.3 d | 69.4 d | 19.4 t | 19.2 t | 75.0 s | 75.0 s | 201.4 s |
| 3 | 22.0 d | 21.9 d | 22.1 d | 22.1 d | 23.6 d | 19.3 d | 20.6 d | 59.0 d | 58.9 d | 102.2 d |
| 4 | 20.7 s | 20.6 s | 20.8 s | 20.8 s | 17.2 s | 16.3 s | 15.1 s | 28.3 t | 28.2 t | 74.5 d |
| 5 | 45.6 d | 45.6 d | 45.8 d | 45.8 d | 46.6 d | 50.1 d | 38.6 d | 37.0 t | 37.0 t | 39.0 t |
| 6 | 29.5 t | 29.5 t | 33.5 t | 33.5 t | 34.7 t | 25.5 t | 27.0 t | 74.4 s | 74.4 s | 72.8 d |
| 7 | 78.2 d | 78.3 d | 76.7 d | 76.9 d | 76.7 d | 35.9 t | 121.1 d | 86.6 d | 86.5 d | 79.7 d |
| 8 | 35.2 d | 35.2 d | 38.3 d | 38.3 d | 38.4 d | 30.7 d | 136.3 s | 23.0 t | 23.0 t | 26.8 t |
| 9 | 142.1 s | 142.2 s | 142.9 s | 143.1 s | 143.5 s | 147.1 s | 50.3 d | 38.6 t | 38.5 t | 127.3 d |
| 10 | 36.5 s | 36.4 s | 36.5 s | 36.5 s | 37.1 s | 37.5 s | 32.4 s | 72.0 s | 71.9 s | 129.3 d |
| 11 | 118.5 d | 118.3 d | 117.7 d | 117.5 d | 117.2 d | 114.4 d | 24.6 t | 76.4 d | 76.3 d | 34.7 t |
| 12 | 37.4 t | 37.4 t | 37.9 t | 38.0 t | 38.1 t | 39.3 t | 37.1 t | 21.2 t | 21.2 t | 52.8 d |
| 13 | 35.2 s | 35.3 s | 35.3 s | 35.4 s | 35.5 s | 35.6 s | 39.8 s | 20.7 t | 20.7 t | 84.4 d |
| 14 | 38.8 t | 38.6 t | 38.9 t | 38.8 t | 38.8 t | 41.8 t | 46.8 t | 76.1 d | 76.1 d | 23.2 t |
| 15 | 68.7 d | 58.9 d | 69.0 d | 59.5 d | 59.6 d | 70.0 d | 76.4 d | 73.3 s | 73.2 s | 11.4 q |
| 16 | 64.4 t | 65.9 t | 64.3 t | 66.0 t | 66.1 t | 64.5 t | 63.8 t | 33.6 t | 33.6 t | |
| 17 | 24.7 q | 24.2 q | 24.8 q | 24.3 q | 24.3 q | 24.9 q | 19.4 q | 25.5 t | 25.4 t | |
| 18 | 18.9 t | 18.8 t | 18.8 t | 18.8 t | 21.7 t | 21.4 t | 19.7 t | 77.7 d | 77.5 d | |
| 19 | 69.6 t | 69.5 t | 69.8 t | 69.8 t | 23.2 q | 24.1 q | 24.6 q | 86.1 s | 86.3 s | |
| 20 | 19.9 q | 19.6 q | 20.0 q | 19.8 q | 19.6 q | 17.9 q | 19.5 q | 32.5 t | 31.9 t | |
| 21 | 26.6 t | 26.8 t | ||||||||
| 22 | 87.5 d | 85.8 d | ||||||||
| 23 | 70.5 s | 82.5 s | ||||||||
| 24 | 24.0 q | 22.1 q | ||||||||
| 25 | 23.7 q | 23.7 q | ||||||||
| 26 | 20.1 q | 20.1 q | ||||||||
| 27 | 21.4 q | 21.4 q | ||||||||
| 28 | 22.9 q | 22.9 q | ||||||||
| 29 | 23.4 q | 23.2 q | ||||||||
| 30 | 27.6 q | 22.4 q | ||||||||
| CH3CO | 21.6 q | 21.5 q | 21.6 q | 21.6 q | 21.6 q | 22.0 q | ||||
| CH3CO | 21.2 q | 21.2 q | 21.1 q | 21.1 q | 20.9 q | |||||
| CH3CO | 21.1 q | 21.1 q | 20.9 q | |||||||
| CH3CO | 20.9 q | |||||||||
| CH3CO | 170.9 s | 170.8 s | 170.9 s | 170.9 s | 170.7 s | 170.4 s | ||||
| CH3CO | 170.6 s | 170.6 s | 170.6 s | 170.7 s | 170.6 s | |||||
| CH3CO | 170.5 s | 170.6 s | 170.5 s | |||||||
| CH3CO | 170.4 s |
Experimental
General
Algal Material
Extraction and Isolation
Acknowledgements
References and Notes
- Masuda, M.; Abe, T.; Suzuki, T.; Suzuki, M. Morphological and chemotaxonomic studies on Laurencia composita and L. okamurae (Ceramiales, Rhodophyta). Phycologia 1996, 35, 550–562. [Google Scholar] [CrossRef]
- Erickson, K. L. Marine Natural Products, Chemical and Biological Perspectives; Scheuer, P.J., Ed.; Academic Press: New York, USA, 1983; Volume 5, pp. 131–257. [Google Scholar]
- Ji, N.Y.; Li, X.M.; Zhang, Y.; Wang, B.G. Two new halogenated chamigrane-type sesquiterpenes and other secondary metabolites from the marine red alga Laurencia okamurai and their chemotaxonomic significance. Biochem. Syst. Ecol. 2007, 35, 627–630. [Google Scholar] [CrossRef]
- Takeda, S.; Kurosawa, E.; Komiyama, K.; Suzuki, T. The structures of cytotoxic diterpenes containing bromine from the marine red alga Laurencia obtusa (Hudson) Lamouroux. Bull. Chem. Soc. Jpn. 1990, 63, 3066–3072. [Google Scholar] [CrossRef]
- Schmitz, F.J.; Michaud, D.P.; Schmidt, P.G. Marine natural products: parguerol, deoxyparguerol, and isoparguerol. New brominated diterpenes with modified pimarane skeletons from the sea hare Aplysia dactylomela. J. Am. Chem. Soc. 1982, 104, 6415–6423. [Google Scholar] [CrossRef]
- Higgs, M.D.; Faulkner, D.J. A diterpene from Laurencia obtusa. Phytochemistry 1982, 21, 789–791. [Google Scholar] [CrossRef]
- Lyakhova, E.G.; Kalinovsky, A.I.; Kolesnikova, S.A.; Vaskovsky, V.E.; Stonik, V.A. Halogenated diterpenoids from the red alga Laurencia nipponica. Phytochemistry 2004, 65, 2527–2532. [Google Scholar] [CrossRef]
- Ji, N.Y.; Li, X.M.; Xie, H.; Ding, J.; Li, K.; Ding, L.P.; Wang, B.G. Highly oxygenated triterpenoids from the marine red alga Laurencia mariannensis (Rhodomelaceae). Helv. Chim. Acta 2008, 91, 1940–1946. [Google Scholar] [CrossRef]
- Suzuki, T.; Suzuki, M.; Furusaki, A.; Matsumoto, T.; Kato, A.; Imanaka, Y.; Kurosawa, E. Teurilene and thyrsiferyl 23-acetate, meso and remarkably cytotoxic compounds from the marine red alga Laurencia obtusa (Hudson) Lamouroux. Tetrahedron Lett. 1985, 26, 1329–1332. [Google Scholar] [CrossRef]
- Suzuki, M.; Kurosawa, E.; Furusaki, A.; Katsuragi, S.-I.; Matsumoto, T. Neolaurallene, a new halogenated C-15 nonterpenoid from the red alga Laurencia okamurai YAMADA. Chem. Lett. 1984, 13, 1033–1034. [Google Scholar]
- Wright, A.D.; König, G.M.; Sticher, O. New sesquiterpenes and C15 acetogenins from the marine red alga Laurencia implicata. J. Nat. Prod. 1991, 54, 1025–1033. [Google Scholar] [CrossRef]
- Rochfort, S.J.; Capon, R.J. Parguerenes revisited: new brominated diterpenes from the southern Australian marine red alga Laurencia filiformis. Aust. J. Chem. 1996, 49, 19–26. [Google Scholar]
- Masuda, M.; Abe, T. The occurrence of Laurencia saitoi Perestenko (L. obtusa auct. japon.) (Ceramiales, Rhodophyta) in Japan. Jpn. J. Phycol. 1993, 41, 7–18. [Google Scholar]
- Kurata, K.; Taniguchi, K.; Agatsuma, Y.; Suzuki, M. Diterpenoid feeding-deterrents from Laurencia saitoi. Phytochemistry 1998, 47, 363–369. [Google Scholar] [CrossRef]
- Suzuki, T.; Takeda, S.; Hayama, N.; Tanaka, I.; Komiyama, K. The structure of brominated diterpene from the marine red alga Laurencia obtusa (Hudson) Lamouroux. Chem. Lett. 1989, 18, 969–970. [Google Scholar]
- Takeda, S.; Matsumoto, T.; Komiyama, K.; Kurosawa, E.; Suzuki, T. A new cytotoxic diterpene from the marine red alga Laurencia obtusa (Hudson) Lamouroux. Chem. Lett. 1990, 19, 277–280. [Google Scholar]
- Takeda, S.; Iimura, Y.; Tanaka, K.; Kurosawa, E.; Suzuki, T. A new naturally occurring racemic compound from the marine red alga Laurencia obtusa (Hudson) Lamouroux. Chem. Lett. 1990, 19, 155–156. [Google Scholar]
- Suzuki, T.; Takeda, S.; Suzuki, M.; Kurosawa, E.; Kato, A.; Imanaka, Y. Cytotoxic squalene-derived polyethers from the marine red alga Laurencia obtusa (Hudson) Lamouroux. Chem. Lett. 1987, 16, 361–364. [Google Scholar]
- Sample Availability: Samples of compounds 1-10 are available from the authors.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ji, N.-Y.; Li, X.-M.; Wang, B.-G. Halogenated Terpenes and a C15-Acetogenin from the Marine Red Alga Laurencia saitoi. Molecules 2008, 13, 2894-2899. https://doi.org/10.3390/molecules13112894
Ji N-Y, Li X-M, Wang B-G. Halogenated Terpenes and a C15-Acetogenin from the Marine Red Alga Laurencia saitoi. Molecules. 2008; 13(11):2894-2899. https://doi.org/10.3390/molecules13112894
Chicago/Turabian StyleJi, Nai-Yun, Xiao-Ming Li, and Bin-Gui Wang. 2008. "Halogenated Terpenes and a C15-Acetogenin from the Marine Red Alga Laurencia saitoi" Molecules 13, no. 11: 2894-2899. https://doi.org/10.3390/molecules13112894
APA StyleJi, N. -Y., Li, X. -M., & Wang, B. -G. (2008). Halogenated Terpenes and a C15-Acetogenin from the Marine Red Alga Laurencia saitoi. Molecules, 13(11), 2894-2899. https://doi.org/10.3390/molecules13112894