A Preliminary Pharmacokinetic Study of Betulin, the Main Pentacyclic Triterpene from Extract of Outer Bark of Birch (Betulae alba cortex)
Abstract
:Introduction
Results and Discussion
Dry triterpene extract


Preliminary pharmacokinetics in rats



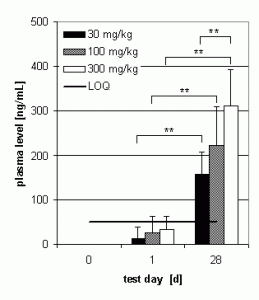
Preliminary pharmacokinetics in dogs


Subchronic toxicity studies
| parameter | intraperitoneal (i.p.) administration to Sprague-Dawley rats | subcutananeous (s.c.) administration to beagle dogs | ||||
|---|---|---|---|---|---|---|
| dose level group | 60 g/kg | 180 g/kg | 540 g/kg | 30 g/kg | 100 g/kg | 300 g/kg |
| leucocytes | n.c. | + 46 % ♀ n.c. ♂ | + 45 % ♀ + 53 % ♂ | n.c. | n.c. | n.c. ♀ + 369 % ♂ |
| platelet count | n.c. | + 31 % ♀ + 34 % ♂ | + 30 % ♀ + 45 % ♂ | n.c. | n.c. | n.c. |
| Differential blood count | n.c. | n.c. | n.c. | n.c. | n.c. | n.c. |
Discussion
Conclusions
Experimental
General
Plant material, extraction and characterization of triterpene extract
Animal studies: Preliminary pharmacokinetics
| administration | animal | suspension medium | dosis schemata | blood sampling |
|---|---|---|---|---|
| intraperitoneal (i.p.) | sprague-dawley rats (3 female, 3 male) per dose level group | sesame oil | 60, 180, 540 g/kg, vol. 10 mL/kg, for 28 days daily | day 1: 0.5, 1, 2, 4 h day 28: 0, 0.5, 1, 2, 4 h |
| subcutan (s.c.) | beagle dogs (3 female, 3 male) per dose level group | PEG 400 / 0.9 % NaCl | 30, 100, 300 mg/kg, vol. 5 mL/kg, for 28 days daily | day 1: 0.5, 1, 2, 4 h day 28: 0, 0.5, 1, 2, 4 h |
Quantification of betulin within plasma samples (GC-MS)
| validation parameter | quantification of BE in plasma |
|---|---|
| specificity | as required |
| linearity | r = 1.000 |
| accuracy | approx. ± 95 % (mean) |
| intra-day- and, inter-day precision | approx. ± 95 % respectively |
| stability | no apparent degradation within 168 h of storage, 24 h of storage in the derivatization reagent |
| recovery | BE: 94.9 ± 4.2 % |
| ER: 71.3 ± 2.8 % | |
| sensitivity, limit of quantification (LOQ) | 0.05 µg/mL |
Subchronic toxicity studies
Statistics
Acknowledgements
References and Notes
- Ekman, R. The suberin monomers and triterpenoids from the outer bark of betula verruosa ehrh. Holzforschung 1983, 37, 205–211. [Google Scholar] [CrossRef]
- Laszczyk, M.; Jäger, S.; Simon-Haarhaus, B.; Scheffler, A.; Schempp, C.M. Physical, chemical and pharmacological characterization of a new oleogel-forming triterpene extract from the outer bark of birch (betulae cortex). Planta Med. 2006, 72, 1389–1395. [Google Scholar] [CrossRef]
- Szuster-Ciesielska, A.; Kandefer-Szerszen, M. Protective effects of betulin and betulinic acid against ethanol-induced cytotoxicity in HepG2 cells. Pharmacol. Rep. 2005, 57, 588–595. [Google Scholar]
- Suksamrarn, S.; Panseeta, P.; Kunchanawatta, S.; Distaporn, T.; Ruktasing, S.; Suksamrarn, A. Ceanothane- and lupane-type triterpenes with antiplasmodial and antimycobacterial activities from Ziziphus cambodiana. Chem. Pharm. Bull. 2006, 54, 535–537. [Google Scholar] [CrossRef]
- Eiznhamer, D.A.; Xu, Z.Q. Betulinic acid: a promising anticancer candidate. IDrugs 2004, 7, 359–373. [Google Scholar]
- Liu, J. Oleanolic acid and ursolic acid: research perspectives. J. Ethnopharmacol. 2005, 100, 92–94. [Google Scholar] [CrossRef]
- Alakurtti, S.; Makela, T.; Koskimies, S.; Yli-Kauhaluoma, J. Pharmacological properties of the ubiquitous natural product betulin. Eur. J. Pharm. Sci. 2006.
- Manez, S.; Recio, M.C.; Giner, R.M.; Rios, J.L. Effect of selected triterpenoids on chronic dermal inflammation. Eur. J. Pharmacol. 1997, 334, 103–105. [Google Scholar] [CrossRef]
- Geetha, T.; Varalakshmi, P. Anti-inflammatory activity of lupeol and lupeol linoleate in rats. J. Ethnopharmacol. 2001, 76, 77–80. [Google Scholar] [CrossRef]
- de la Puerta, R.; Martinez-Dominguez, E.; Ruiz-Gutierrez, V. Effect of minor components of virgin olive oil on topical antiinflammatory assays. Z. Naturforsch. 2000, 55, 814–819. [Google Scholar]
- Cichewicz, R.H.; Kouzi, S.A. Chemistry, Biological Activity, and Chemotherapeutic Potential of Betulinic Acid for the Prevention and Treatment of Cancer and HIV Infections. Med. Res. Rev. 2004, 24, 90–114. [Google Scholar] [CrossRef]
- Astudillo, L.; Rodriguez, J.A.; Schmeda-Hirschmann, G. Gastroprotective activity of oleanolic acid derivatives on experimentally induced gastric lesions in rats and mice. J. Pharm. Pharmacol. 2002, 54, 583–588. [Google Scholar] [CrossRef]
- Liu, J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Singh, G.B.; Singh, S.; Bani, S.; Gupta, B.D.; Banerjee, S.K. Anti-inflammatory activity of oleanolic acid in rats and mice. J. Pharm. Pharmacol. 1992, 44, 456–458. [Google Scholar] [CrossRef]
- Udeani, G.O.; Zhao, G.M.; Shin, Y.G.; Cooke, B.P.; Graham, J.; Beecher, C.W.W.; Kinghorn, A.D.; Pezzuto, J.M. Pharmacokinetics and tissue distribution of betulinic acid in CD-1 mice. Biopharm. Drug Dispos. 1999, 20, 379–383. [Google Scholar] [CrossRef]
- Jäger, S.; Winkler, K.; Pfüller, U.; Scheffler, A. Solubility Studies of Oleanolic Acid and Betulinic Acid in Aqueous Solutions and Plant extracts of Viscum album L. Planta Med. 2007, 73, 157–162. [Google Scholar] [CrossRef]
- Jeong, D.W.; Kim, Y.H.; Kim, H.H.; Ji, H.Y.; Yoo, S.D.; Choi, W.R.; Lee, S.M.; Han, C.K.; Lee, H.S. Dose-linear pharmacokinetics of oleanolic acid after intravenous and oral administration in rats. Biopharm. Drug Dispos. 2007, 28, 51–57. [Google Scholar] [CrossRef]
- Strickley, R.G. Solubilizing excipients in oral and injectable formulations. Pharm. Res. 2004, 21, 201–230. [Google Scholar] [CrossRef]
- Huyke, C.; Laszczyk, M.; Scheffler, A.; Ernst, R.; Schempp, C.M. Treatment of actinic keratoses with birch bark extract: a pilot study. J. Dtsch. Dermatol. Ges. 2006, 4, 132–136. [Google Scholar] [CrossRef]
- Huyke, C.; Reuter, J.; Rodig, M.; Kersten, A.; Laszczyk, M.; Scheffler, A.; Nashan, D.; Schempp, C. Treatment of actinic keratoses with a novel betulin-based oleogel. A prospective, randomized, comparative pilot study. J. Dtsch. Dermatol. Ges. 2008. Sep 19. [Epub]. [Google Scholar]
- Ying, Q.L.; Rinehart, A.R.; Simon, S.R.; Cheronis, J.C. Inhibition of human leucocyte elastase by ursolic acid. Evidence for a binding site for pentacyclic triterpenes. Biochem. J. 1991, 277, 521–526. [Google Scholar]
- Cheng, X.; Shin, Y.G.; Levine, B.S.; Smith, A.C.; Tomaszewski, J.E.; van Breemen, R.B. Quantitative analysis of betulinic acid in mouse, rat and dog plasma using electrospray liquid chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2003, 17, 2089–2092. [Google Scholar] [CrossRef]
- Shin, Y.G.; Cho, K.H.; Chung, S.M.; Graham, J.; Das Gupta, T.K.; Pezzuto, J.M. Determination of betulinic acid in mouse blood, tumor and tissue homogenates by liquid chromatography-electrospray mass spectrometry. J. Chromatogr. B 1999, 732, 331–336. [Google Scholar] [CrossRef]
- Wright, J.H. A rapid method for the differential staining of blood films and malaria parasites. J. Med. Res. 1902, 7, 138. [Google Scholar]
- Sample Availability: Samples of the compound TE are available from authors.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jäger, S.; Laszczyk, M.N.; Scheffler, A. A Preliminary Pharmacokinetic Study of Betulin, the Main Pentacyclic Triterpene from Extract of Outer Bark of Birch (Betulae alba cortex). Molecules 2008, 13, 3224-3235. https://doi.org/10.3390/molecules13123224
Jäger S, Laszczyk MN, Scheffler A. A Preliminary Pharmacokinetic Study of Betulin, the Main Pentacyclic Triterpene from Extract of Outer Bark of Birch (Betulae alba cortex). Molecules. 2008; 13(12):3224-3235. https://doi.org/10.3390/molecules13123224
Chicago/Turabian StyleJäger, Sebastian, Melanie N. Laszczyk, and Armin Scheffler. 2008. "A Preliminary Pharmacokinetic Study of Betulin, the Main Pentacyclic Triterpene from Extract of Outer Bark of Birch (Betulae alba cortex)" Molecules 13, no. 12: 3224-3235. https://doi.org/10.3390/molecules13123224
APA StyleJäger, S., Laszczyk, M. N., & Scheffler, A. (2008). A Preliminary Pharmacokinetic Study of Betulin, the Main Pentacyclic Triterpene from Extract of Outer Bark of Birch (Betulae alba cortex). Molecules, 13(12), 3224-3235. https://doi.org/10.3390/molecules13123224