The Use of Coumarins as Environmentally-Sensitive Fluorescent Probes of Heterogeneous Inclusion Systems
Abstract
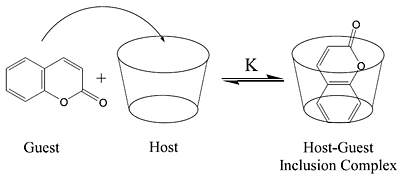
:Introduction

Coumarin Photophysics



Coumarins as Fluorescent Guests Included in Molecular Hosts

Cyclodextrins



Cucurbiturils

Other Molecular Hosts
Coumarins as Fluorescent Probes of Micelles

Coumarins as Fluorescent Probes of Polymer Hosts
Coumarins as Fluorescent Probes of Solid Host Materials
Porous Solid Hosts
Coumarin-Doped Glasses and Crystals
Other Solid Host Materials
Other Heterogeneous Systems
Summary
Acknowledgements
References and Notes
- Sethna, S.M.; Shah, N.M. The Chemistry of Coumarins. Chem. Rev. 1945, 36, 1–62. [Google Scholar]
- Song, P.-S.; Gordon, W.H. III A Spectroscopic Study of the Excited States of Coumarin. J. Phys. Chem. 1970, 74, 4234–4240. [Google Scholar] [CrossRef]
- Woods, L.L.; Shamma, S.M. Synthesis of Substituted Coumarins with Fluorescent Properties. J. Chem. Eng. Data 1971, 16, 101–102. [Google Scholar] [CrossRef]
- Jones, G. II; Jackson, W.R.; Kanoktanaporn, S.; Halper, A.M. Solvent Effects on Photophysical Parameters for Coumarin Laser Dyes. Opt. Commun. 1980, 33, 315–320. [Google Scholar] [CrossRef]
- Jones, G., II; Jackson, W.R.; Choi, C.; Bergmark, W.R. Solvent Effects on Emission Yield and Lifetime for Coumarin Laser Dyes. Requirements for a Rotatory Decay Mechanism. J. Phys. Chem. 1985, 89, 294–300. [Google Scholar] [CrossRef]
- Nag, A.; Bhattacharyya, K. Role of Twisted Intramolecular Charge Transfer in the Fluorescence Sensitivity of Biological Probes: Diethylaminocoumarin Laser Dyes. Chem. Phys. Lett. 1990, 169, 12–16. [Google Scholar] [CrossRef]
- Patalakha, N.S.; Yufit, D.S.; Kirpichenok, M.A.; Gordeeva, N.A.; Struchkov, Yu.T.; Grandberg, I.I. Luminescence-Spectral and Acid-Base Characteristics of 3-Aryl-7-Diethylaminocoumarins. Khim. Geterot. Soed. 1991, 1, 40–46. [Google Scholar]
- Gordeeva, N.A.; Kirpichënok, M.A.; Patalakha, N.S.; Khutorretskii, V.M.; Grandberg, I.I. Spectral, Spectral-Luminescence and Acid-Base Properties of 3-Fluoro-7-Diethylaminocoumarins. Khim. Geterot. Soed. 1991, 5, 619–624. [Google Scholar]
- Abdel-Mottaleb, M.S.A.; Antonious, M.S.; Abo Ali, M.M.; Ismail, L.F.M.; El Sayed, B.A.; Sherief, A.M.K. Photophysics and Dynamics of Coumarin Laser Dyes and their Analytical Implications. Proc. Indian Acad. Sci. 1992, 104, 185–196. [Google Scholar]
- Yip, R.W.; Wen, Y.-X.; Szabo, A.G. Decay Associated Fluorescence Spectra of Coumarin 1 and Coumarin 102: Evidence for a Two-State Solvation Kinetics in Organic Solvents. J. Phys. Chem. 1993, 97, 10458–10462. [Google Scholar] [CrossRef]
- López Arbeloa, T.; López Arbeloa, F.; Tapia, M.J.; López Arbeloa, I. Hydrogen-Bonding Effect on the Photophysical Properties of 7-Aminocoumarin Derivatives. J. Phys. Chem. 1993, 97, 4704–4707. [Google Scholar] [CrossRef]
- Taneja, L.; Sharma, A.K.; Singh, R.D. Study of Photophysical Properties of Coumarins: Substituent and Concentration Dependence. J. Luminesc. 1995, 63, 203–214. [Google Scholar] [CrossRef]
- Królicki, R.; Jarzęba, W.; Mostafavi, M.; Lampre, I. Preferential Solvation of Coumarin 153 - The Role of Hydrogen Bonding. J. Phys. Chem. A. 2002, 106, 1708–1713. [Google Scholar] [CrossRef]
- El-Kemary, M.; Rettig, W. Multiple Emission in Coumarins with Heterocyclic Substituents. Phys. Chem. Chem. Phys. 2003, 5, 5221–5228. [Google Scholar] [CrossRef]
- Moog, R.S; Kim, D.D.; Oberle, J.J.; Ostrowski, S.G. Solvent Effects on Electronic Transitions of Highly Dipolar Dyes: A Comparison of Three Approaches. J. Phys. Chem. A. 2004, 108, 9294–9301. [Google Scholar]
- Dahiya, P.; Kumbhakar, M.; Mukherjee, T.; Pal., H. Effect of Protic Solvents on Twisted Intramolecular Charge Transfer State Formation in Coumarin-152 and Coumarin-481 Dyes. Chem. Phys. Lett. 2005, 414, 148–154. [Google Scholar] [CrossRef]
- Bayrakçeken, F.; Yaman, A.; Hayvali, M. Photophysical and Photochemical Study of Laser-Dye Coumarin-481 and its Photoproduct in Solution. Spectrochim. Acta A 2005, 61, 983–987. [Google Scholar] [CrossRef]
- Barik, A.; Kumbhakar, M.; Nath, S.; Pal, H. Evidence for the TICT Mediated Nonradiative Deexcitation Process for the Excited Coumarin-1 Dye in High Polarity Protic Solvents. Chem. Phys. 2005, 315, 277–285. [Google Scholar] [CrossRef]
- Sharma, V.K.; Saharo, P.D.; Sharma, N.; Rastogi, R.C.; Ghoshal, S.K.; Mohan, D. Influence of Solvent and Substituent on Excited State Characteristics of Laser Grade Coumarin Dyes. Spectrochim. Acta A 2003, 59, 1161–1170. [Google Scholar] [CrossRef]
- Grabowski, Z.R.; Rotkiewicz, K. Structural Changes Accompanying Intramolecular Electron Transfer: Focus on Twisted Intramolecular Charge-Transfer States and Structures. Chem. Rev. 2003, 103, 3899–4031. [Google Scholar] [CrossRef]
- Muthuramu, K.; Ramamurthy, V. 7-Alkoxy Coumarins as Fluorescence Probes for Microenvironments. J. Photochem. 1984, 26, 57–64. [Google Scholar] [CrossRef]
- Heldt, J.R.; Heldt, J.; Stoń, M.; Diehl, H.A. Photophysical Properties of 4-Alkyl- and 7-Alkoxycoumarin Derivatives. Absorption and Emission Spectra, Fluorescence Quantum Yield and Decay Time. Spectrochim. Acta A 1995, 51, 1549–1563. [Google Scholar] [CrossRef]
- Moeckli, P. Preparation of Some New Red Fluorescent 4-Cyanocoumarin Dyes. Dyes Pigments 1980, 1, 3–15. [Google Scholar]
- Richard, J.-A.; Massonneau, M.; Renard, P.-Y.; Romieu, A. 7-Hydroxycoumarin-Hemicyanine Hybrids: A New Class of Far-Red Emitting Fluorogenic Dyes. Org. Lett. 2008, 10, 4175–4178. [Google Scholar] [CrossRef]
- Ismail, L.F.M.; Antonious, M.S.; Mohamed, H.A.; Abdel-Hay Ahmed, H. Fluorescence Properties of Some Coumarin Dyes and their Analytical Implication. Proc. Indian Acad. Sci. 1992, 104, 331–338. [Google Scholar]
- Christie, R.M.; Liu, C.-H. Studies of Fluorescent Dyes: Part 2. An Investigation of the Synthesis and Electronic Spectral Properties of Substituted 3-(2’-Benzimidazoyl)coumarins. Dyes Pigments 2000, 47, 79–89. [Google Scholar] [CrossRef]
- Sui, G.; Kele, P.; Orbulescu, J.; Huo, Q.; Leblanc, R.M. Synthesis of a Coumarin Based Fluorescent Amino Acid. Lett. Pept. Sci. 2002, 8, 47–51. [Google Scholar]
- Turki, H.; Abid, S.; El Gharbi, R.; Fery-Forgues, S. Optical Properties of New Fluorescent Iminocoumarins. Part 2. Solvatochromic Study and Comparison with the Corresponding Coumarin. C.R. Chimie 2006, 9, 1252–1259. [Google Scholar] [CrossRef]
- Woods, L.L.; Fooladi, M. 3,3’-Keto Biscoumarins. J. Chem. Eng. Data 1967, 12, 624–626. [Google Scholar] [CrossRef]
- Ammar, H.; Fery-Forgues, S.; El Gharbi, R. UV/Vis Absorption and Fluorescence Spectroscopic Study of Novel Symmetrical Biscoumarin Dyes. Dyes Pigments 2003, 57, 259–265. [Google Scholar] [CrossRef]
- McCarthy, P.K.; Blanchard, G.J. AM1 Study of the Electronic Structure of Coumarins. J. Phys. Chem. 1993, 97, 12205–12209. [Google Scholar] [CrossRef]
- Christie, R.M.; Liu, C.-H. Studies of Fluorescent Dyes: Part 1. An Investigation of the Electronic Spectral Properties of Substituted Coumarins. Dyes Pigments 1999, 42, 85–93. [Google Scholar] [CrossRef]
- Nemkovich, N.A.; Baumann, W.; Reis, H.; Zvinevich, Yu.V. Electro-optical and Laser Spectrofluorimetry Study of Coumarins 7 and 30: Evidence for the Existence of the Close-Lying Electronic States and Conformers. J. Photochem. Photobiol. AChem. 1997, 109, 287–292. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and General Overview of Cyclodextrin Chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef]
- Hedges, A.R. Industrial Applications of Cyclodextrins. Chem. Rev. 1998, 98, 2035–2044. [Google Scholar] [CrossRef]
- Takadate, A.; Fujino, H.; Goya, S.; Hirayama, F.; Otagiri, M.; Uekama, K.; Yamaguchi, H. Fluorescence Behavior of 7-Substituted Coumarin Derivatives by Inclusion Complexation with Beta- Cyclodextrin. Yakugaku Sasshi J. Pharm. Soc. Jap. 1983, 103, 193–197. [Google Scholar]
- Scypinksi, S.; Drake, J.M. Photophysics of Coumarin Inclusion Complexes with Cyclodextrin. Evidence for Normal and Inverted Complex Formation. J. Phys. Chem. 1985, 89, 2432–2435. [Google Scholar]
- Bergmark, W.R.; Davis, A.; York, C.; Macintosh, A.; Jones, G. II Dramatic Fluorescence Effects for Coumarin Laser Dyes Coincluded with Organic Solvents in Cyclodextrins. J. Phys. Chem. 1990, 94, 5020–5022. [Google Scholar] [CrossRef]
- Nowakowska, M.; Smoluch, M.; Sendor, D. The Effect of Cyclodextrins on the Photochemical Stability of 7-Amino-4-methylcoumarin in Aqueous Solution. J. Inclus. Phenom. Macro. Chem. 2001, 40, 213–219. [Google Scholar] [CrossRef]
- Al-Kindy, S.M.Z.; Suliman, F.E.O.; Al-Hamadi, A.A. Fluorescence Enhancement of Coumarin-6-sulfonyl Chloride Amino Acid Derivatives in Cyclodextrin Media. Anal. Sci. 2001, 17, 539–547. [Google Scholar] [CrossRef]
- Dondon, R.; Fery-Forgues, S. Inclusion Complex of Fluorescent 4-Hydroxycoumarin Derivatives with Native β-Cyclodextrin: Enhanced Stabilization Induced by the Appended Substituent. J. Phys. Chem. B 2001, 105, 10715–10722. [Google Scholar] [CrossRef]
- Douhal, A. Ultrafast Guest Dynamics in Cyclodextrin Nanocavities. Chem. Rev. 2004, 104, 1955–1976. [Google Scholar] [CrossRef]
- Vajda, S.; Jiminez, R.; Rosenthal, S.J.; Fidler, V.; Fleming, G.R.; Castner, E.W., Jr. Femtosecond to Nanosecond Solvation Dynamics in Pure Water and inside the γ-Cyclodextrin Cavity. J. Chem. Soc. Faraday Trans. 1995, 91, 867–873. [Google Scholar] [CrossRef]
- Roy, D.; Mondal, S.K.; Sahu, K.; Ghosh, S.; Sen, P.; Bhattacharyya, K. Temperature Dependence of Anisotropy Decay and Solvation Dynamics of Coumarin 153 in γ-Cyclodextrin Aggregates. J. Phys. Chem. A 2005, 109, 7359–7364. [Google Scholar] [CrossRef]
- Wagner, B.D.; Fitzpatrick, S.J.; McManus, G.J. Fluorescence Suppression of 7-Methoxycoumarin upon Inclusion into Cyclodextrins. J. Inclus. Phenom. Macro. Chem. 2003, 47, 187–192. [Google Scholar]
- Sen, P.; Roy, D.; Mondal, S.K.; Sahu, K.; Ghosh, S.; Bhattacharyya, K. Fluorescence Anisotropy Decay and Solvation Dynamics in a Nanocavity: Coumarin 153 in Methyl β–Cyclodextrins. J. Phys. Chem. A 2005, 109, 9716–9722. [Google Scholar] [CrossRef]
- Velic, D.; Knapp, M.; Köhler, G. Supramolecular Inclusion Complexes Between a Coumarin Dye and β-Cyclodextrin, and Attachment Kinetics of a Thiolated β-Cyclodextrin to Gold Surface. J. Mol. Struct. 2001, 598, 49–56. [Google Scholar] [CrossRef]
- Velic, D.; Köhler, G. Supramolecular Surface Layer: Coumarin/Thiolated Cyclodextrin/Gold. Chem. Phys. Lett. 2003, 371, 483–489. [Google Scholar] [CrossRef]
- Tablet, C.; Hillebrand, M. Theoretical and Experimental Study of the Inclusion Complexes of the 3-Carboxy-5,6-Benzocoumarinic Acid with Cyclodextrins. Spectrochim. Acta A 2008, 70, 740–748. [Google Scholar] [CrossRef]
- Chakraborty, A.; Seth, D.; Chakrabarty, D.; Sarkar, N. Photoinduced Intermolecular Electron Transfer from Dimethyl Aniline to 7-Amino Coumarin Dyes in the Surface of β-Cyclodextrin. Spectrochim. Acta A 2006, 64, 801–808. [Google Scholar] [CrossRef]
- Moorthy, J.N.; Venkatesan, K.; Weiss, R.G. Photodimerization of Coumarins in Solid Cyclodextrin Inclusion Complexes. J. Org. Chem. 1992, 57, 3292–3297. [Google Scholar] [CrossRef]
- Brett, T.J.; Alexander, J.M.; Clark, J.L.; Ross, C.R. II; Harbison, G.S.; Stezowski, J.J. Chemical Insight from Crystallographic Disorder: Structural Studies of a Supramolecular β-Cyclodextrin/Coumarin Photochemical System. Chem. Commun. 1999, 1275–1276. [Google Scholar]
- Brett, T.J.; Alexander, J.M.; Stezowski, J.J. Chemical Insight from Crystallographic Disorder-Structural Studies of Supramolecular Photochemical Systems. Part 2. The β-Cyclodextrin-4,7-Dimethylcoumarin Inclusion Complex: a New β-Cyclodextrin Dimer Packing Type, Unanticipated Photoproduct Formation, and an Examination of Guest Influence on β-CD Dimer Packing. J. Chem. Soc., Perkin Trans. 2000, 2, 1095–1103. [Google Scholar]
- Lagona, J.; Mukhopadhyay, P.; Chakrabarti, S.; Isaacs, L. The Cucurbit[n]uril Family. Angew. Chem. Int. Ed. 2005, 44, 4844–4870. [Google Scholar] [CrossRef]
- Freeman, W.A.; Mock, W.L.; Shih, N.Y. Cucurbituril. J. Am. Chem. Soc. 1981, 103, 7367–7368. [Google Scholar] [CrossRef]
- Kim, J.; Jung, I.-S.; Kim, S.-Y.; Lee, E.; Kang, J.-K.; Sakamoto, S.; Yamaguchi, K.; Kim, K. New Cucurbituril Homologues: Synthesis, Isolation, Characterization, and X-ray Crystal Structures of Cucurbit[n]uril (n=5,7, and 8). J. Am. Chem. Soc. 2000, 122, 540–541. [Google Scholar] [CrossRef]
- Nau, W.M.; Mohanty, J. Taming Fluorescent Dyes with Cucurbituril. Int. J. Photoenergy 2005, 7, 133–141. [Google Scholar] [CrossRef]
- Barooah, N.; Pemberton, B.C.; Johnson, A.C.; Sivaguru, J. Photodimerization and Complexation Dynamics of Coumarins in the Presence of Cucurbit[8]urils. Photochem. Photobiol. Sci. 2008, 7, 1473–1479. [Google Scholar] [CrossRef]
- Frauchiger, L.; Shirota, H.; Uhrich, K.E.; Castner, E.W., Jr. Dynamic Fluorescence Probing of the Local Environments within Amphiphilic Starlike Macromolecules. J. Phys. Chem. B 2002, 106, 7463–7468. [Google Scholar]
- Steege, K.E.; Wang, J.; Uhrich, K.E.; Castner, E.W. Jr. Local Polarity and Microviscosity in the Hydrophobic Cores of Amphiphilic Star-like and Scorpion-like Macromolecules. Macromolecules 2007, 40, 3739–3748. [Google Scholar] [CrossRef]
- Shirota, H.; Segawa, H. Time-Resolved Fluorescence Study on Liquid Oligo(ethylene oxide)s: Coumarin 153 in Poly(ethylene glycol)s and Crown Ethers. J. Phys. Chem. A 2003, 107, 3719–3727. [Google Scholar] [CrossRef]
- Marques, A.D.S.; Marques, G.S.S. Spectroscopic Studies on Coumarin in Micelles. Photochem. Photobiol. 1994, 59, 153–160. [Google Scholar] [CrossRef]
- Al-Kindy, S.Z.M.; El-Sherbini, S.A.; Abdel-Kader, M.H. UV-visible and Fluorescence Characteristics of the Luminescent Label Coumarin-6-sulphonyl Chloride in Homogeneous and Micellar Solutions. Anal. Chem. Acta 1994, 285, 329–333. [Google Scholar] [CrossRef]
- Dutt, G.B. Are the Experimentally Determined Microviscosities of the Micelles Probe Dependent? J. Phys. Chem. B 2004, 108, 3651–3657. [Google Scholar] [CrossRef]
- Hara, K.; Baden, N.; Kajimoto, O. Pressure Effects on Water Solvation Dynamics in Micellar Media. J. Phys.: Condens. Matter 2004, 16, S1207–S1214. [Google Scholar] [CrossRef]
- Hara, K.; Kuwabara, H.; Kajimoto, O. Pressure Effect on Solvation Dynamics in Micellar Environment. J. Phys. Chem. A 2001, 105, 7174–7179. [Google Scholar] [CrossRef]
- Baden, N.; Kajimoto, O.; Hara, K. High-Pressure Studies on Aggregation Number of Surfactant Micelles Using the Fluorescence Quenching Method. J. Phys. Chem. A 2002, 106, 8621–8624. [Google Scholar]
- Molina-Bolívar, J.A.; Aguiar, J.; Carnero Ruiz, C. Light Scattering and Fluorescence Probe Studies on Micellar Properties of Triton X-100 in KCl Solutions. Mol. Phys. 2001, 99, 1729–1741. [Google Scholar] [CrossRef]
- Carnero Ruiz, C.; Molina-Bolívar, J.A.; Aguiar, J.; MacIsaac, G.; Moroze, S.; Palepu, R. Thermodynamic and Structural Studies of Triton X-100 in Micelles in Ethylene Glycol-Water Mixed Solvents. Langmuir 2001, 17, 6831–6840. [Google Scholar] [CrossRef]
- Molina-Bolívar, J.A.; Aguiar, J.; Peula-García, J.M.; Carnero Ruiz, C. Photophysical and Light Scattering Studies on the Aggregation Behavior of Triton X-100 in Formamide-Water Mixed Solvents. Mol. Phys. 2002, 100, 3259–3269. [Google Scholar] [CrossRef]
- Kumbhakar, M.; Nath, S.; Mukherjee, T.; Pal, H. Solvation Dynamics in Triton X-100 and Triton X-165 Micelles: Effect of Micellar Size and Hydration. J. Chem. Phys. 2004, 121, 6026–6033. [Google Scholar] [CrossRef]
- Kumbhakar, M.; Goel, T.; Mukherjee, T.; Pal, H. Effect of Lithium Chloride in the Palisade Layer of the Triton X-100 Micelle: Two Sites for Lithium Ions as Revealed by Solvation and Rotational Dynamics Studies. J. Phys. Chem. A 2005, 109, 18528–18534. [Google Scholar]
- Seth, D.; Chakroborty, A.; Setua, P.; Sarkar, N. Interaction of Ionic Liquid with Water in Ternary Microemulsions (Triton X-100/Water/1-Butyl-3-methylimidazolium Hexafluorophosphate) Probed by Solvent and Rotational Relaxation of Coumarin 153 and Coumarin 151. Langmuir 2006, 22, 7768–7775. [Google Scholar] [CrossRef]
- Dondon, R.; Bertorelle, F.; Fery-Forgues, S. 4-Hydroxycoumarin Derivatives in Micelles: An Approach to Detect a Structural Transition Using with Fluorescent Viscosity Probes. J. Fluoresc. 2002, 12, 163–165. [Google Scholar] [CrossRef]
- Shirota, H.; Tamoto, Y.; Segawa, H. Dynamic Fluorescence Probing of the Microenvironment of Sodium Dodecyl Sulfate Micelle Solutions: Surfactant Concentration Dependence and Solvent Isotope Effect. J. Phys. Chem. A 2004, 108, 3244–3252. [Google Scholar] [CrossRef]
- De Paula, R.; da Hora Machado, A.E.; de Miranda, J.A. 3-Benzoxazol-2-yl-7-(N,N-diethylamino)-chromen-2-one as a Fluorescence Probe for the Investigation of Micellar Microenvironments. J. Photochem. Photobiol. A Chem. 2004, 165, 109–114. [Google Scholar] [CrossRef]
- Dutt, G.B. Comparison of the Microenvironments of Aqueous Sodium Dodecyl Sulfate Micelles in the Presence of Inorganic and Organic Salts: A Time-Resolved Fluorescence Anisotropy Approach. Langmuir 2005, 21, 10391–10397. [Google Scholar] [CrossRef]
- Pantano, D.A.; Sonoda, M.T.; Skaf, M.S.; Laria, D. Solvation of Coumarin 314 at Water/Air Interfaces Containing Anionic Surfactants. I. Low Coverage. J. Phys. Chem. B 2005, 109, 7365–7372. [Google Scholar] [CrossRef]
- Kumbhakar, M.; Goel, T.; Nath, S.; Mukherjee, T.; Pal, H. Microenvironment in the Corona Region of Triblock Copolymer Micelles: Temperature Dependent Solvation and Rotational Relaxation Dynamics of Coumarin Dyes. J. Phys. Chem. B 2006, 110, 25646–25655. [Google Scholar] [CrossRef]
- Kumbhakar, M.; Ganguly, R. Influence of Electrolytes on the Microenvironment of F127 Triblock Copolymer Micelles: A Solvation and Rotational Dynamics Study of Coumarin Dyes. J. Phys. Chem. B 2007, 111, 3935–3842. [Google Scholar] [CrossRef]
- Kumbhakar, M. Effect of Ionic Surfactants on the Hydration Behavior Triblock Copolymer Micelles: A Solvation Dynamics Study of Coumarin 153. J. Phys. Chem. B 2007, 111, 12154–12161. [Google Scholar] [CrossRef]
- Satpati, A.K.; Kumbhakar, M.; Nath, S.; Pal, H. Roles of Diffusion and Activation Barrier on the Appearance of Marcus Inversion Behavior: A Study of a Photoinduced Electron-Transfer Reaction in Aqueous Triblock Copolymer (P123) Micellar Solutions. J. Phys. Chem. B 2007, 111, 7550–7560. [Google Scholar]
- Kumbhakar, M. Aggregation of Ionic Surfactants to Block Copolymer Assemblies: A Simple Fluorescence Spectral Study. J. Phys. Chem. B 2007, 111, 14250–14255. [Google Scholar] [CrossRef]
- Singh, P.K.; Kumbhakar, M.; Pal, H.; Nath, S. Effect of Electrostatic Interaction on the Location of Molecular Probe in Polymer-Surfactant Supramolecular Assembly: A Solvent Relaxation Study. J. Phys. Chem. B 2008, 111, 7771–7777. [Google Scholar]
- Grant, C.D.; DeRitter, M.R.; Steege, K.E.; Fadeeva, T.A.; Castner, E.W. Jr. Fluorescence Probing of Interior, Interfacial, and Exterior Regions in Solution Aggregates of Poly(ethylene oxide)-Poly(propylene oxide)-Poly(ethylene oxide) Triblock Copolymers. Langmuir 2005, 21, 1745–1752. [Google Scholar] [CrossRef]
- Grant, C.D.; Steege, K.E.; Bunagan, M.R.; Castner, E.W., Jr. Microviscosity in Multiple Regions of Complex Aqueous Solutions of Poly(ethylene oxide)-Poly(propylene oxide)-Poly(ethylene oxide). J. Phys. Chem. B 2005, 109, 22273–22284. [Google Scholar] [CrossRef]
- Ghosh, S.; Dey, S.; Adhikari, A.; Mandal, U.; Bhattacharyya, K. Ultrafast Fluorescence Resonance Energy Transfer in the Micelle and the Gel Phase of a PEO-PPO-PEO Triblock Copolymer: Excitation Wavelength Dependence. J. Phys. Chem. B 2007, 111, 7085–7091. [Google Scholar] [CrossRef]
- Aguiar, J.; Molina-Bolívar, J.A.; Peula-García, J.M.; Carnero Ruiz, C. Thermodynamics and Micellar Properties of Tetradecyltrimethylammonium Bromide in Formamide-Water Mixtures. J. Coll. Interf. Sci. 2002, 255, 382–390. [Google Scholar] [CrossRef]
- Chakraborty, A.; Charkrabarty, D.; Hazra, P.; Seth, D.; Sarkar, N. Photoinduced Intermolecular Electron Transfer Between Coumarin Dyes and Electron Scavenging Solvents in Cetyltrimethylammonium Bromide (CTAB) Micelles: Evidence for Marcus Inverted Region. Chem. Phys. Lett. 2003, 382, 508–517. [Google Scholar] [CrossRef]
- Tamoto, Y.; Segawa, H.; Shirota, H. Solvation Dynamics in Aqueous Anionic and Cationic Micelle Solutions: Sodium Alkyl Sulfate and Alkyltrimethylammonium Bromide. Langmuir 2005, 21, 3757–4764. [Google Scholar] [CrossRef]
- Carnero Ruiz, C.; Molina-Bolívar, J.A.; Aguiar, J.; McIsaac, G.; Moroze, S.; Palepu, R. Effect of Ethylene Glycol on the Thermodynamic and Micellar Properties of Tween 20. Colloid Polym. Sci. 2003, 281, 531–541. [Google Scholar] [CrossRef]
- Chakraborty, A.; Seth, D.; Charkrabarty, D.; Setua, P.; Sarkar, N. Dynamics of Solvent and Rotational Relaxation of Coumarin 153 in Room-Temperature Ionic Liquid 1-Butyl-3-methylimidazolium Hexafluorophosphate Confined in Brij-35 Micelles: A Picosecond Time-Resolved Fluorescence Spectroscopic Study. J. Phys. Chem. A 2005, 109, 11110–11116. [Google Scholar]
- Riter, R.E.; Willard, D.M.; Levinger, N.E. Water Immobilization at Surfactant Interfaces in Reverse Micelles. J. Phys. Chem. B 1998, 102, 2705–2714. [Google Scholar] [CrossRef]
- Levinger, N.E. Ultrafast Dynamics in Reverse Micelles, Microemulsions, and Vesicles. Curr. Opin. Coll. Interface Sci. 2001, 5, 118–124. [Google Scholar] [CrossRef]
- Raju, B.B.; Costa, S.M.B. Excited-State Behavior of 7-Dimethylaminocoumarin Dyes in AOT Reversed Micelles: Size Effects. J. Phys. Chem. B 1999, 103, 4309–4317. [Google Scholar] [CrossRef]
- Raju, B.B.; Costa, S.M.B. Nanosecond Time Resolved Emission Spectroscopy of Aminocoumarins in AOT Reversed Micelles. Phys. Chem. Chem. Phys. 1999, 1, 5029–5034. [Google Scholar] [CrossRef]
- Hazra, P.; Sarkar, N. Solvation Dynamics of Coumarin 490 in Methanol and Acetonitrile Reverse Micelles. Phys. Chem. Chem. Phys. 2002, 4, 1040–1045. [Google Scholar] [CrossRef]
- Hazra, P.; Charkrabarty, D.; Sarkar, N. Solvation Dynamics of Coumarin 152A in Methanol and Acetonitrile Reverse Micelles. Chem. Phys. Lett. 2002, 358, 523–530. [Google Scholar] [CrossRef]
- Hazra, P.; Charkrabarty, D.; Sarkar, N. Solvation Dynamics of Coumarin 153 in Aqueous and Non-Aqueous Reverse Micelles. Chem. Phys. Lett. 2003, 371, 553–562. [Google Scholar] [CrossRef]
- Chakraborty, A.; Seth, D.; Charkrabarty, D.; Hazra, P.; Sarkar, N. Photoinduced Electron Transfer from Dimethylaniline to Coumarin Dyes in Reverse Micelles. Chem. Phys. Lett. 2005, 405, 18–25. [Google Scholar] [CrossRef]
- Seth, D.; Charkrabarty, D.; Chakraborty, A.; Sarkar, N. Study of Energy Transfer from 7-Amino Coumarin Donors to Rhodamine 6G Acceptor in Non-Aqueous Reverse Micelles. Chem. Phys. Lett. 2005, 401, 546–532. [Google Scholar] [CrossRef]
- Yamasaki, T.; Kajimoto, O.; Hara, K. High-Pressure Studies on AOT Reverse Micellar Aggregate by Fluorescence Probe Method. J. Photochem. Photobiol. A: Chem. 2003, 156, 145–150. [Google Scholar] [CrossRef]
- Choudhury, S.D.; Kumbhakar, M.; Nath, S.; Sarkar, S.K.; Mukherjee, T.; Pal, H. Compartmentalization of Reactants in Different Regions of Sodium 1,4-Bis(2-ethylhexyl)sulfosuccinate/Heptane/Water Reverse Micelles and Its Influence on Bimolecular Electron-Transfer Kinetics. J. Phys. Chem. B 2007, 111, 8842–8853. [Google Scholar] [CrossRef]
- Mitra, R.K.; Sinha, S.S.; Pal, S.K. Temperature-Dependent Solvation Dynamics of Water in Sodium Bis(2-ethy;lhexyl)sulfosuccinate/Isooctane Reverse Micelles. Langmuir 2008, 24, 49–56. [Google Scholar] [CrossRef]
- Narayanan, S.S.; Sinha, S.S.; Sarkar, R.; Pal, S.K. Picosecond to Nanosecond Reorganization of Water in AOT/Lecithin Mixed Reverse Micelles of Different Morphology. Chem. Phys. Lett. 2008, 452, 99–104. [Google Scholar] [CrossRef]
- Biswas, R.; Rohman, N.; Pradhan, T.; Buchner, R. Intramolecular Charge Transfer Reaction, Polarity, and Dielectric Relaxation in AOT/Water/Heptane Reverse Micelles: Pool Size Dependence. J. Phys. Chem. B 2008, 112, 9379–9388. [Google Scholar] [CrossRef]
- Bekiari, V.; Lianos, P. Photophysical Studies in AOT Films Deposited on Fused Silica Slides. J. Coll. Interface Sci. 1996, 183, 552–558. [Google Scholar] [CrossRef]
- Hof, M.; Lianos, P. Structural Studies of Thin AOT Films by Using the Polarity Fluorescent Probe Coumarin-153. Langmuir 1997, 13, 290–294. [Google Scholar] [CrossRef]
- Trenor, S.R.; Shultz, A.R.; Love, B.J.; Long, T.E. Coumarins in Polymers: From Light Harvesting to Photo-Cross-Linkable Tissue Scaffolds. Chem. Rev. 2004, 104, 3059–3077. [Google Scholar] [CrossRef]
- Prabhugouda, M.; Lagare, M.T.; Mallikarjuna, N.N.; Naidu, B.V.K.; Aminabhavi, T.M. Energy Transfer Processes Between Primary and Secondary Dopants in Polystyrene Solutions Dissolved in 1,4-Dioxane. J. Appl. Polym. Sci. 2005, 95, 336–341. [Google Scholar] [CrossRef]
- Corrales, T.; Abrusi, C.; Peinado, C.; Catalina, F. Fluorescent Sensor as Physical Amplifier of Chemiluminescence: Application to the Study of Poly(ethylene terephthalate). Macromolecules 2004, 37, 6596–6605. [Google Scholar] [CrossRef]
- Mason, M.D.; Ray, K.; Pohlers, G.; Cameron, J.F.; Grober, R.D. Probing the Local pH of Polymer Photoresist Films Using a Two-Color Single Molecule Nanoprobe. J. Phys. Chem. B 2003, 107, 14219–14224. [Google Scholar] [CrossRef]
- Frenette, M.; Ivan, M.G.; Scaiano, J.C. Use of Fluorescent Probes to Determine Catalytic Chain Length in Chemically Amplifies Resists. Can. J. Chem. 2005, 83, 869–874. [Google Scholar] [CrossRef]
- Oh, J.K.; Stöeva, V.; Rademacher, J.; Farwaha, R.; Winnik, M.A. Synthesis, Characterization, and Emulsion Polymerization of Polymerizable Coumarin Derivatives. J. Polym. Sci: A: Polym. Chem. 2004, 42, 3479–3489. [Google Scholar] [CrossRef]
- Corrent, S.; Hahn, P.; Pohlers, G.; Connolly, T.J.; Scaiano, J.C.; Fornés, V.; Garcia, H. Intrazeolite Photochemistry. 22. Acid-Base Properties of Coumarin 6. Characterization in Solution, the Solid State, and Incorporated into Supramolecular Systems. J. Phys. Chem. B 1998, 102, 5852–5858. [Google Scholar] [CrossRef]
- Kamijo, T.; Yamaguchi, A.; Suzuki, S.; Teramae, N.; Itoh, T.; Ikeda, T. Solvation Dynamics of Coumarin 153 in Alcohols Confined in Silica Nanochannels. J. Phys. Chem. A 2008, 112, 11535–11542. [Google Scholar] [CrossRef]
- Zhao, W.; Li, D.; He, B.; Zhang, J.; Huang, J.; Zhang, L. The Photoluminescence of Coumarin Derivative Encapsulated in MCM-41 and Ti-MCM-41. Dyes Pigments 2005, 64, 265–270. [Google Scholar] [CrossRef]
- Li, D.; Zhao, W.; Sun, X.; Zhang, J.; Anpo, M.; Zhao, J. Photophysical Properties of Coumarin Derivative Incorporated in MCM-41. Dyes Pigments 2006, 68, 33–37. [Google Scholar] [CrossRef]
- Wlordarczyk, P.; Komarneni, S.; Roy, R.; White, W.B. Enhanced Fluorescence of Coumarin Laser Dye in the Restricted Geometry of a Porous Nanocomposite. J. Mater. Chem. 1996, 6, 1967–1969. [Google Scholar] [CrossRef]
- Gruzinskii, V.V.; Kukhto, A.V.; Mozalev, A.M.; Surganov, V.F. Luminescence Properties of Anodic Aluminum Oxide Films with Organic Luminophores Embedded into Pores. J. Appl. Spectrosc. 1997, 64, 497–502. [Google Scholar] [CrossRef]
- Dunn, B.; Zink, J.I. Optical Properties of Sol-Gel Glasses Doped with Organic Molecules. J. Mater. Chem. 1991, 1, 903–913. [Google Scholar] [CrossRef]
- Keeling-Tucker, T.; Brennan, J.D. Fluorescent Probes as Reporters on the Local Structure and Dynamics in Sol-Gel-Derived Nanocomposite Materials. Chem. Mater. 2001, 13, 3331–3350. [Google Scholar] [CrossRef]
- Takahashi, Y.; Shimada, R.; Maeda, A.; Kojima, K.; Uchida, K. Photophysics of Coumarin 4 Doped-Amorphous Silica Glasses Prepared by the Sol-Gel Method. J. Luminesc. 1996, 68, 187–192. [Google Scholar] [CrossRef]
- Takahashi, Y.; Maeda, A.; Kojima, K.; Uchida, K. Luminescence of Dyes Doped in Sol-Gel Coating Film. J. Luminesc. 2000, 87-89, 767–769. [Google Scholar] [CrossRef]
- Oh, E.O.; Gupta, R.K.; Whang, C.M. Effects of pH and Dye Concentration on the Optical and Structural Properties of Coumarin 4 Dye-Doped SiO2-PDMS Xerogels. J. Sol-Gel Sci. Tech. 2003, 28, 279–288. [Google Scholar] [CrossRef]
- Ferrer, M.L.; del Monte, F.; Levy, D. Microviscosities of Silica Gel-Glasses and Ormosils through Fluorescence Anisotropy. J. Phys. Chem. B 2001, 103, 11076–11080. [Google Scholar] [CrossRef]
- Baumann, R.; Ferrante, C.; Kneuper, E.; Deeg, F.-W.; Bräuchle, C. Influence of Confinement on the Solvation and Rotational Dynamics of Coumarin 153 in Ethanol. J. Phys. Chem. A 2003, 107, 2422–2430. [Google Scholar] [CrossRef]
- Deshpande, A.V.; Panhalkar, R.R. Spectroscopic Properties of Coumarin 2 in HCl and HNO3 Catalyzed Sol-Gel Glasses. J. Luminesc. 2002, 96, 185–193. [Google Scholar] [CrossRef]
- Fukushima, M.; Yanagi, H.; Hayashu, S.; Suganuma, N.; Taniguchi, Y. Fabrication of Gold Nanoparticles and their Influence on Optical Properties of Dye-Doped Sol-Gel Films. Thin Solid Films 2003, 438-439, 39–43. [Google Scholar] [CrossRef]
- Unger, B.; Rurack, K.; Müller, R.; Resch-Genger, U.; Buttke, K. Effects of the Sol-Gel Processing on the Fluorescence Properties of Laser Dyes in Tetraethoxysilane Derived Matrices. J. Sol-Gel Sci. Tech. 2000, 19, 799–802. [Google Scholar] [CrossRef]
- Deshpande, A.V.; Kumar, U. Molecular Forms of Coumarin-307 in Sol-Gel Glasses. J. Fluoresc. 2006, 16, 679–687. [Google Scholar] [CrossRef]
- Suratwala, T.; Gardlund, Z.; Davidson, K.; Uhlmann, D.R. Photostability of Silylated Coumarin Dyes in Polyceram Hosts. J. Sol-Gel Sci. Tech. 1997, 8, 973–978. [Google Scholar]
- Suratwala, T.; Gardlund, Z.; Davidson, K.; Uhlmann, D.R. Silylated Coumarin Dyes in Sol-Gel Hosts. 1. Structure and Environmental Factors of Fluorescent Properties. Chem. Mater. 1998, 10, 190–198. [Google Scholar] [CrossRef]
- Suratwala, T.; Gardlund, Z.; Davidson, K.; Uhlmann, D.R. Silylated Coumarin Dyes in Sol-Gel Hosts. 2. Photostability and Sol-Gel Processing. Chem. Mater. 1998, 10, 199–209. [Google Scholar] [CrossRef]
- Ganschow, M.; Hellriegel, C.; Kneuper, E.; Wark, M.; Thiel, C.; Schulz-Ekloff, G.; Bräuchle, C.; Wöhrle, D. Panchromatic Chromophore Mixtures in an AlPO4-5 Molecular Sieve: Spatial Separation Effects and Energy Transfer Cascades. Adv. Funct. Mater. 2004, 14, 269–276. [Google Scholar] [CrossRef]
- Galian, R.E.; Laferrièrre, M.; Scaiano, J.C. Doping of Photonic Crystal Fibers with Fluorescent Probes: Possible Functional Materials for Optrode Sensors. J. Mater. Chem. 2006, 16, 1697–1701. [Google Scholar] [CrossRef]
- Aloisi, G.G.; Costantino, U.; Elisei, F.; Latterini, L.; Natali, C.; Nocchette, M. Preparation and Photo-physical Characterization of Nanocomposites Obtained by Intercalation and Co-intercalation of Organic Chromophores into Hydrotalcite-Like Compounds. J. Mater. Chem. 2002, 12, 3316–3323. [Google Scholar] [CrossRef]
- Fujii, K.; Iyi, N.; Sasai, R.; Hayashi, S. Preparation of a Novel Luminous Heterogeneous System: Rhodamine/Coumarin/Phyllosilicate Hybrid with Blue Shift in Fluorescence Emission. Chem. Mater. 2008, 20, 2994–3002. [Google Scholar] [CrossRef]
- Fischer, K.; Prause, S.; Spange, S.; Cichos, F.; Von Borczyskowski, C. Surface Polarity of Cellulose Derivatives Observed by Coumarin 151 and Coumarin 153 As Solvatochromic and Fluorochromic Probes. J. Polymer Sci.: B: Polymer Phys. 2003, 41, 1210–1218. [Google Scholar] [CrossRef]
- Guan, H.; Zhu, L.; Zhou, H.; Tang, H. Rapid Probing of Photocatalytic Activity on Titania-Based Self-Cleaning Materials using 7-Hydroxycoumarin Fluorescent Probe. Anal. Chim. Acta 2008, 608, 73–78. [Google Scholar] [CrossRef]
- Dutta, A.K.; Ray, K.; Misra, T.N. Aggregation Induced Reabsorption of 3-(2-Benzothiazolyl)-7-Octadecyloxy Coumarin Molecules Absorbed in Langmuir-Blodgett Films: A Fluorescence Study. Solid State Commun. 1995, 94, 53–59. [Google Scholar] [CrossRef]
- Ray, K.; Dutta, A.K.; Misra, T.N. Spectroscopic Properties of 3-(2-Benzothiazolyl)-7-Octadecyloxy Coumarin in Langmuir-Blodgett Films. J. Luminesc. 1997, 71, 123–130. [Google Scholar] [CrossRef]
- Fox, M.A.; Li, W.; Wooten, M.; McKerrow, A.; Whiteshell, J.K. Fluorescence Probes for Chemical Reactivity at the Interface of a Self-Assembled Monolayer. Thin Solid Films 1998, 327-329, 477–480. [Google Scholar] [CrossRef]
- Nicol, E.; Habib-Jiwan, J.-L.; Jonas, A.M. Polyelectrolyte Multilayers as Nanocontainers for Functional Hydrophilic Molecules. Langmuir 2003, 19, 6178–6186. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wagner, B.D. The Use of Coumarins as Environmentally-Sensitive Fluorescent Probes of Heterogeneous Inclusion Systems. Molecules 2009, 14, 210-237. https://doi.org/10.3390/molecules14010210
Wagner BD. The Use of Coumarins as Environmentally-Sensitive Fluorescent Probes of Heterogeneous Inclusion Systems. Molecules. 2009; 14(1):210-237. https://doi.org/10.3390/molecules14010210
Chicago/Turabian StyleWagner, Brian D. 2009. "The Use of Coumarins as Environmentally-Sensitive Fluorescent Probes of Heterogeneous Inclusion Systems" Molecules 14, no. 1: 210-237. https://doi.org/10.3390/molecules14010210
APA StyleWagner, B. D. (2009). The Use of Coumarins as Environmentally-Sensitive Fluorescent Probes of Heterogeneous Inclusion Systems. Molecules, 14(1), 210-237. https://doi.org/10.3390/molecules14010210