Screening of Agelasine D and Analogs for Inhibitory Activity against Pathogenic Protozoa; Identification of Hits for Visceral Leishmaniasis and Chagas Disease
Abstract
:Introduction
Results and Discussion
Antiprotozoal activities

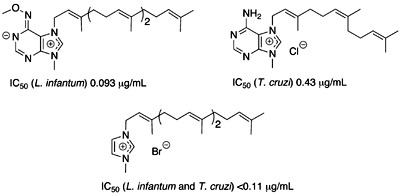
Structure – activity relationships
| Compound No. a | P. falciparum | L. infantum | T. cruzi | T. b. brucei | MRC-5 | ||||
|---|---|---|---|---|---|---|---|---|---|
| IC50μg/mLb(<0.2 μg/mL)c | SId(>20)c | IC50μg/mLe(<0.5 μg/mL)c | SId(>20)c | IC50μg/mf(<1.0 μg/mL)c | SId(>50)c | IC50 μg/mLg(<0.2 μg/mL)c | SId(>100)c | IC50 μg/mL | |
| 1a | 0.46 | 1.9 | 7.5 | <1 | <0.079 | >11 | 1.9 | <1 | 0.86 |
| 1b | 0.63 | <1 | 0.77 | <1 | <0.096 | >3.6 | 0.20 | 1.8 | 0.35 |
| 1c | 0.74 | <1 | 0.99 | <1 | <0.11 | >2.8 | <0.11 | >2.8 | 0.31 |
| 1d | 0.10 | 20 | 0.093 | 22 | 0.11 | 18 | 0.23 | 8.7 | 2.0 |
| 1e | 4.2 | <1 | 5.4 | <1 | 1.4 | 2.3 | 0.29 | 11 | 3.2 |
| 1f | 0.26 | 1.0 | 0.27 | <1 | <0.12 | >2.2 | <0.12 | >2.2 | 0.26 |
| 1g | 5.3 | <1 | 12 | <1 | 0.81 | <1 | 1.0 | <1 | 0.49 |
| 1h | 10 | <1 | 18 | <1 | 3.0 | 1.2 | 1.7 | 2.1 | 3.5 |
| 1i | 3.5 | <1 | 1.2 | <1 | 1.3 | <1 | 0.29 | 3.2 | 0.92 |
| 1j | 0.30 | <1 | 0.23 | <1 | <0.11 | >1.9 | 0.11 | 1.9 | 0.21 |
| 1k | 7.3 | <1 | 1.3 | <1 | <0.13 | >2.2 | <0.13 | >2.2 | 0.28 |
| 1l | 1.3 | <1 | 12 | <1 | 0.14 | 3.8 | <0.13 | >4.1 | 0.53 |
| 1m | 0.69 | <1 | 0.99 | <1 | <0.12 | >4 | <0.12 | >4 | 0.48 |
| 1n | 0.29 | 16 | 0.63 | 7.1 | 0.49 | 9.2 | 0.30 | 15 | 4.5 |
| 1o | 0.94 | <1 | 4.0 | <1 | <0.12 | >3.8 | <0.12 | >3.8 | 0.45 |
| 2a | 2.9 | >9.0 | >26 | _ | 0.43 | >60 | 13 | >2 | >26 |
| 2b | 0.96 | <1 | 2.9 | <1 | <0.12 | >6.3 | 0.23 | 3.3 | 0.75 |
| 2c (Agelasine D) | 0.29 | 23 | 1.5 | 4.5 | 4.5 | 1.5 | 0.90 | 7.4 | 6.7 |
| 3a | 10 | 1.1 | >26 | <1 | 2.5 | 4.4 | 2.3 | 4.8 | 11 |
| 3b | 3.9 | 3.1 | >29 | <1 | 3.6 | 3.3 | 2.6 | 4.6 | 12 |
| 3c | 1.8 | <1 | 2.7 | <1 | <0.13 | >2.4 | <0.13 | >2.4 | 0.31 |
| 3d | 2.3 | 1.1 | >36 | <1 | 2.2 | 1.1 | 1.2 | 2.1 | 2.5 |
| 3e | 3.5 | 6 | >44 | <1 | 4.5 | 3.3 | 1.3 | 12 | 15 |
| 3f | >29 | <1 | >29 | <1 | 0.77 | 3.5 | 2.7 | 1.0 | 2.7 |
| 3g | >26 | _ | 9.8 | >2.7 | 0.97 | >27 | 2.9 | >9.0 | >26 |
| 4a | 9.4 | <1 | >26 | <1 | 2.3 | 1.1 | 3.5 | <1 | 2.6 |
| 4b | >28 | <1 | >28 | <1 | 0.28 | 7.5 | 0.89 | 2.4 | 2.1 |
| 4c | 25 | <1 | >33 | <1 | 1.5 | 1.0 | 3.8 | <1 | 1.5 |
| 5 | 0.97 | 7.8 | <0.11 | >69 | <0.11 | >69 | <0.11 | >69 | 7.6 |
| 6 | 1.0 | 2.0 | 2.5 | >69 | <0.12 | >17 | 0.19 | 11 | 2.0 |
| 7 | 10 | 1.6 | >32 | >69 | 11 | 1.5 | 4.1 | 3.9 | 16 |
| 8 | 3.37 | >69 | 13 | >69 | 0.19 | 12 | 0.63 | 3.7 | 2.3 |
| 9 | >28 | _ | >28 | _ | 3.2 | >8.8 | 14 | >2 | >28 |
Conclusions
Experimental
Compounds
Test plate production
Biological screening tests
Antiplasmodial activity
Antitrypanosomal activity
Antileishmanial activity
Cytotoxicity assay
Acknowledgements
References and Notes
- Gelb, M.H. Drug discovery for malaria: a very challenging and timely endeavor. Curr. Opin. Chem. Biol. 2007, 11, 440–445. [Google Scholar] [CrossRef]
- Tuteja, R. Malaria – an overview. FEBS J. 2007, 274, 4670–4679. [Google Scholar] [CrossRef]
- Croft, S.L.; Barrett, M.P.; Urbina, J.A. Chemotherapy of trypanosomiases and leishmaniasis. Trends Parasitol. 2005, 21, 508–512. [Google Scholar] [CrossRef]
- Chappuis, F.; Sundar, S.; Hailu, A.; Ghalib, H.; Rijal, S.; Peeling, R.W.; Alvar, J.; Boerlaert, M. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nature. Rev. Microbiol. 2007, 5, S7–S16. [Google Scholar] [CrossRef]
- Barrett, M.P.; Boykin, D.W.; Brun, R.; Tidwell, R.R. Human African trypanosomiasis: pharmacological re-engagement with a neglected disease. J. Pharmacol. 2007, 152, 1155–1171. [Google Scholar]
- Teixeira, A.R.L.; Nitz, N.; Giumaro, M.C.; Gomes, C.; Santos-Buch, C.A. Chagas disease. Postgrad. Med. J. 2006, 82, 788–798. [Google Scholar] [CrossRef]
- Nwaka, S.; Hudson, A. Innovative lead discovery strategies for tropical diseases. Nature Rev. Drug. Discov. 2006, 5, 941–955. [Google Scholar] [CrossRef]
- Plubrukarn, A. Marine invertebrates: a diverted approach towards the treatment of tropical diseases. Recent. Prog. Med. Plants 2006, 11, 439–461. [Google Scholar]
- Capon, R.J.; Faulkner, D.J. Antimicrobial metabolites from a Pacific sponge, Agelas sp. J. Am. Chem. Soc. 1984, 106, 1819–1822. [Google Scholar] [CrossRef]
- Nakamura, H.; Wu, H.; Ohizumi, Y.; Hirata, Y. Agelasine-A –B, -C and –D, novel bicyclic diterpenoids with a 9-methyladeninium unit possessing inhibitory effects on Na,K-ATPase isolated from the Okinawian sea sponge Agelas sp. Tetrahedron Lett. 1984, 25, 2989–2992. [Google Scholar] [CrossRef]
- Wu, H.; Nakamura, H.; Kobayashi, J.; Ohizumi, Y. Agelasine-E and –F, novel monocyclic diterpenoids with a 9-methyladeninium unit possessing inhibitory effects on Na,K-ATPase isolated from the Okinawian sea sponge Agelas nakamurai Hoshino. Tetrahedron Lett. 1984, 25, 3719–3722. [Google Scholar] [CrossRef]
- Wu, H.; Nakamura, H.; Kobayashi, J.; Kobayashi, M.; Ohizumi, Y.; Hirata, Y. Structures of agelasines, diterpenes having a 9-methyladeninium chromophore isolated from the Okinavian marine sponge Agelas nakamurai Hoshino. Bull. Chem. Soc. Jpn. 1986, 59, 2495–2504. [Google Scholar] [CrossRef]
- Ishida, K.; Ishibashi, M.; Shigemori, H.; Sasaki, T.; Kobayashi, J. Agelasine G, a new antileukemic alkaloid from the Okinavian marina sponge Agelas sp. Chem. Pharm. Bull. 1992, 40, 766–767. [Google Scholar] [CrossRef]
- Hattori, T.; Adachi, K.; Shizuri, Y. New agelasine compound from the marine sponge Agelas mauretania as an antifouling substance against macroalgae. J. Nat. Prod. 1997, 60, 411–413. [Google Scholar] [CrossRef]
- Fu, X.; Schmitz, F.J.; Tanner, R.S.; Kelly-Borges, M. Agelasines H and I, 9-methyladenine-containing diterpenoids from Agelas sponge. J. Nat. Prod. 1998, 61, 548–550. [Google Scholar] [CrossRef]
- Iwagawa, T.; Kaneko, M.; Okamura, H.; Nakatani, M.; van Soest, R.W.M. New alkaloids from the Papua New Guinean sponge Agelas nakamurai. J. Nat. Prod. 1998, 61, 1310–1312. [Google Scholar] [CrossRef]
- Appenzeller, J.; Michi, G.; Martin, M.-T.; Gallard, J.-F.; Menou, J.-L.; Boury-Esnault, N.; Hooper, J.; Petek, S.; Chevalley, S.; Valentin, A.; Zaparucha, A.; Al-Mourabit, A.; Debitus, C. Agelasines J, K, and L from the Solomon Islands marine sponge Agelas cf. mauritiana. J. Nat. Prod. 2008, 71, 1451–1454. [Google Scholar] [CrossRef]
- Utenova, B.T.; Gundersen, L.-L. Synthesis of (+)-agelasine D from (+)-manool. Tetrahedron Lett. 2004, 45, 4233–4235. [Google Scholar] [CrossRef]
- Vik, A.; Hedner, E.; Charnock, C.; Samuelsen, Ø.; Larsson, R.; Gundersen, L.-L.; Bohlin., L. (+)-Agelasine D: improved synthesis and evaluation of antibacterial and cytotoxic activities. J. Nat. Prod. 2006, 69, 381–386. [Google Scholar] [CrossRef]
- Bakkestuen, A.K.; Gundersen, L.-L.; Petersen, D.; Utenova, B.T.; Vik, A. Synthesis and antimycobacterial activity of agelasine E and analogs. Org. Biol. Chem. 2005, 3, 1025–1033. [Google Scholar] [CrossRef]
- Proszenyák, Á; Brændvang, M.; Charnock, C.; Gundersen, L.-L. The first synthesis of ent-Agelasine F. Tetrahedron 2009, 65, 194–199. [Google Scholar] [CrossRef]
- Proszenyák, Á; Charnock, C.; Hedner, E.; Larsson, R.; Bohlin, L.; Gundersen, L.-L. Synthesis and antimicrobial and antineoplastic activities of agelasine and agelasimine analogs with a β-cyclocitral derived substituent. Arch. Pharm. Chem. Life Sci. 2007, 340, 625–634. [Google Scholar] [CrossRef]
- Vik, A.; Hedner, E.; Charnock, C.; Tangen, L.W.; Samuelsen, Ø.; Larsson, R.; Bohlin., L.; Gundersen, L.-L. Antimicrobial and cytotoxic activity of agelasine and agelasimine analogs. Bioorg. Med. Chem. 2007, 15, 4016–4037. [Google Scholar] [CrossRef]
- Roggen, H.; Gundersen, L.-L. Synthetic studies directed towards agelasine analogs. Synthesis, tautomerism, and alkylation of 2-substituted N-methoxy-9-methyl-9H-purin-6-amines. Eur. J. Org. Chem. 2008, 5099–5106. [Google Scholar] [CrossRef]
- Sjögren, M.; Dahlström, M.; Hedner, E.; Jonsson, P.R.; Vik, A.; Gundersen, L.-L.; Bohlin, L. Antifouling activity of the sponge metabolite agelasine D and synthesized analogs on Balanus improvisus. Biofouling 2008, 24, 251–258. [Google Scholar] [CrossRef]
- Fathi-Afshar, R.; Allen, T.M. Biological active metabolites from Agelas mauretania. Can. J. Chem. 1988, 66, 45–50. [Google Scholar] [CrossRef]
- Fathi-Afshar, R.; Allen, T.M. Some pharmacological activities of novel adenine-related compounds isolated from a marine sponge Agelas mauretania. Can. J. Physiol. Pharmacol. 1989, 67, 276–281. [Google Scholar] [CrossRef]
- TDR, Lead discovery for drugs, Business Plan 2008-2013. 2007; BL 3.
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar]
- Makler, M.T.; Ries, J.M.; Williams, J.A.; Bancroft, J.E.; Piper, R.C.; Gibbins, B.L.; Hinrichs, D.J. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am. J. Trop. Med. Hyg. 1993, 48, 739–741. [Google Scholar]
- Hirumi, H.; Hirumi, K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 1989, 75, 985–989. [Google Scholar] [CrossRef]
- Raz, B.; Iten, M.; Grether-Buhler, Y.; Kaminsky, R.; Brun, R. The Alamar Blue asssay to determine drug sensitivity of African trypanosomes (T. b. rhodesiense, T. b. gambiense) in vitro. Acta Trop. 1997, 68, 139–147. [Google Scholar] [CrossRef]
- Buckner, F.S.; Verlinde, C.L.; La Flamme, A.C.; Van Voorhis, W.C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob. Agents Chemother. 1996, 40, 2592–2597. [Google Scholar]
- Sample Availability: Some samples are available from the corresponding author.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vik, A.; Proszenyák, Á.; Vermeersch, M.; Cos, P.; Maes, L.; Gundersen, L.-L. Screening of Agelasine D and Analogs for Inhibitory Activity against Pathogenic Protozoa; Identification of Hits for Visceral Leishmaniasis and Chagas Disease. Molecules 2009, 14, 279-288. https://doi.org/10.3390/molecules14010279
Vik A, Proszenyák Á, Vermeersch M, Cos P, Maes L, Gundersen L-L. Screening of Agelasine D and Analogs for Inhibitory Activity against Pathogenic Protozoa; Identification of Hits for Visceral Leishmaniasis and Chagas Disease. Molecules. 2009; 14(1):279-288. https://doi.org/10.3390/molecules14010279
Chicago/Turabian StyleVik, Anders, Ágnes Proszenyák, Marieke Vermeersch, Paul Cos, Louis Maes, and Lise-Lotte Gundersen. 2009. "Screening of Agelasine D and Analogs for Inhibitory Activity against Pathogenic Protozoa; Identification of Hits for Visceral Leishmaniasis and Chagas Disease" Molecules 14, no. 1: 279-288. https://doi.org/10.3390/molecules14010279
APA StyleVik, A., Proszenyák, Á., Vermeersch, M., Cos, P., Maes, L., & Gundersen, L. -L. (2009). Screening of Agelasine D and Analogs for Inhibitory Activity against Pathogenic Protozoa; Identification of Hits for Visceral Leishmaniasis and Chagas Disease. Molecules, 14(1), 279-288. https://doi.org/10.3390/molecules14010279