Pentacyclic Triterpene Distribution in Various Plants – Rich Sources for a New Group of Multi-Potent Plant Extracts
Abstract
:Introduction

| Triterpene family | Triterpene | R1 | R2 | M [g/mol] | Abbreviation |
|---|---|---|---|---|---|
| lupane | lupeol | CH3 | 426.70 | LU | |
| betulin | CH2OH | 442.72 | BE | ||
| betulinic acid | COOH | 456.71 | BA | ||
| oleanane | β-amyrin | CH3 | H | 426.70 | bAM |
| erythrodiol | CH2OH | H | 442.72 | ER | |
| oleanolic acid | COOH | H | 456.71 | OA | |
| maslinic acid | COOH | OH | 472.70 | MA | |
| ursane | α-amyrin | CH3 | 426.70 | aAM | |
| uvaol | CH2OH | 442.72 | UV | ||
| ursolic acid | COOH | 456.71 | UA |
Results and Discussion
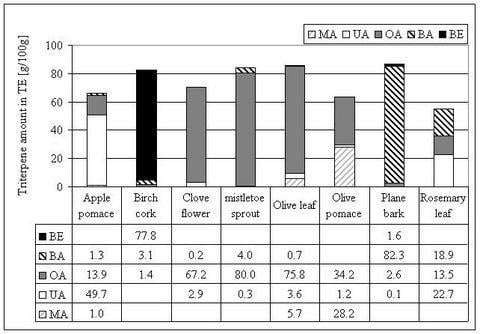
Triterpene distribution within various plant materials

| Common name | Plant part | [g/100 g dry matter] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LU | BE | BA | bAM | ER | OA | aAM | UV | UA | ||
| horse-chestnut | leaves | det | ||||||||
| aloe vera | leaves | 0.10 | ||||||||
| bearberry | leaves | 0.29 | 0.12 | 0.10 | 0.18 | 0.27 | 0.25 | 0.35 | 1.24 (0.40–0.75) | |
| birch | bark | (0.9–2.1) | (10.5–18.3) | (0.5–1.3) | (0.2–0.4) | (0.1–1.1) | ||||
| pot marigold | flowers | det | det (2.0) | |||||||
| common centaury | herb | det | 0.16 | det | ||||||
| coffee | leaves | det | 1.80 | |||||||
| european cornel | leaves | 0.15 | ||||||||
| hawthorn | leaves, flowers | 0.10 | 0.52 | |||||||
| euca-lyptus | leaves | 0.84 | 0.31 | 1.17 | ||||||
| lavender | leaves | 0.13 | 0.45 | 1.59 | ||||||
| lavender | flowers | 0.12 | 0.40 | 1.05 | ||||||
| 11 diff. apples | fruit peel | det+ | 0.28 (0.07) | 1.43 (0.3–0.7) | ||||||
| apple pomace | pomace | 0.16 | 0.80 | |||||||
| lemon balm | leaves | 0.16 | 0.67 | |||||||
| oleander | leaves | 0.11 | 0.37 | 1.27 | ||||||
| sweet basil | leaves | det | det (0.3) | |||||||
| olive | leaves | det (0.3) | 3.10 (1.3) | (0.3) | 0.18 (0.5) | |||||
| olive | bark | det+ | det+ | det+ | 0.98+ | det+ | ||||
| olive | fruit* | 0.21 (0.09–0.16) | ||||||||
| olive | pomace | 0.18 | ||||||||
| marjoram | leaves | 0.19 | 0.66 | |||||||
| oregano | leaves | det | 0.28 | |||||||
| anisseed | seed | det | ||||||||
| greater plantain | leaves | det | 0.21 | |||||||
| planes | bark | det+ | 2.44 (3.3) | det+ | ||||||
| sour cherry | unripe fruit | det | ||||||||
| pear williams' | fruit peel | 0.20 | ||||||||
| rosemary | leaves | 1.53 (0.61) | 1.23 (0.91) | 2.95 (1.58) | ||||||
| sage | leaves | (0.02) | det | 0.67 (0.76) | 1.80 (1.52) | |||||
| black elder | leaves | 0.12 | 0.58 | |||||||
| black elder | bark | det | det | 0.08 | 0.32 | |||||
| winter savory | leaves | (0.04) | 0.14 (0.54) | 0.49 (0.09) | ||||||
| tomato | fruit peel | det | ||||||||
| lilac | leaves | det | ||||||||
| clove | flower | det+ | 1.65 | det+ | ||||||
| common thyme | leaves | 0.37 | 0.94 | |||||||
| common vervain | herb | det | 0.17 | |||||||
| mistletoe | sprouts | det (0.05) | 0.86 (0.16) | |||||||
| grape vine | leaves | det | ||||||||
| Apple | OA per apple [mg] | UA per apple [mg] |
|---|---|---|
| Jonagold | 0.018 | 0.100 |
| Jonagored | 0.010 | 0.058 |
| Royal Gala 1 | 0.009 | 0.052 |
| Royal Gala 2 | 0.008 | 0.038 |

Preparation of triterpene dry extracts (TE)

Conclusions
Experimental
General
| Binomial name | Common name | Plant part | Plant origin, identification; batch or collection |
|---|---|---|---|
| Aesculus hippocastanum | horse-chestnut | leaves | collection *, D-75223 Niefern; collection: 06.08.07 |
| Aloe vera | aloe vera | leaf | greenhouse *, D-75223 Niefern; collection: 06.08.07 |
| Arctostaphylos uva-ursi | bearberry | leaves | Linden Apotheke, D-75223 Niefern; batch: 22.06.07 |
| Calendula officinalis, | pot marigold | flowers | Linden Apotheke, D-75223 Niefern; batch: 05/2009 |
| Centaurium erythraea | common centaury | herb | Linden Apotheke, D-75223 Niefern; batch: 22.06.07 |
| Coffea | coffee | leaves | greenhouse*, D-75223 Niefern; collection: 13.07.07 |
| Cornus mas | european cornel | leaves | collection *, D-75223 Niefern; collection: 06.08.07 |
| Crataegus | hawthorn | leaves & flowers | Linden Apotheke, D-75223 Niefern; batch: 22.06.07 |
| Eucalyptus | eucalyptus | leaves | Heinrich Klenk, D-97525 Schwebheim; batch: 2011 A 060411 01 |
| Lavandula angustifolia | lavender | leaves | collection *, D-75223 Niefern; collection: 06.08.07 |
| Lavandula angustifolia | lavender | flowers | collection *, D-75177 Pforzheim; collection: June 08 |
| Malus domestica | apple | peel | brettacher, collection *, D-72805 Lichtenstein; collection: autumn 06 |
| M. domestica | apple | peel | jonagored, E. Grundler, D-78333 Espasingen; batch: L 20 111 |
| M. domestica | apple | peel | jonagold, Salemfrucht, D-88682 Salem; batch: L 24218 |
| M. domestica | apple | peel | granny smith, Plus Warenhaus, 45466 Mühlheim; batch: August 07 |
| M. domestica | apple | peel | golden delicious, Plus Warenhaus, 45466 Mühlheim; batch: August 07 |
| M. domestica | apple | peel | royal gala 1, OGM Richard Schsugg, D-88097 Eriskirch-Wolfzennen; batch: 17.08.07 |
| M. domestica | apple | peel | royal gala 2, Clementi Gabr. GmbH Leifers-Südtirol; batch: 17.08.07 |
| M. domestica | apple | peel | royal gala 3, Südtiroler Apfel g.g.A. VOG Gen.landw.Ges. I – 39018 Terlan, batch: 17.08.07 |
| M. domestica | apple | peel | belle de boskoop, collection *, D-76534 Geroldsau; collection: 30.09.08 |
| M. domestica | apple | peel | yellow transparent, collection *, D-75223 Niefern; collection: October 08 |
| M. domestica | apple | peel | delba, collection *, D-75203 Königsbach; collection*: July 07 |
| M. domestica | apple | pomace | Herbstreith & Fox, D-75305 Neuenbürg; Herbavital F12 batch: 14.08.07 |
| Melissa officinalis | lemon balm | leaves | collection *, D-75223 Niefern; collection: 06.08.07 |
| Nerium oleander | oleander | leaves | collection *, D-75446 Wiernsheim; collection: 19.04.07 |
| Ocimum basilicum | sweet basil | leaves | Zielpunkt Warenhandel, Plus Warenhaus, 45466 Mühlheim; batch: MHD 2009 |
| Olea europeae | olive | leaves | collection **, Greece; collection: 01.07.07 |
| Olea europeae | olive | bark | collection **, Greece; collection: 01.07.07 |
| Olea europeae | olive | fruit | without endocarp, Las Cuarenta, Plus Warenhaus, D-45466 Mühlheim; batch: L-05/02/2010 |
| Olea europeae | olive | pomace | Merum Verlag, I-51035 Lamporecchio; batch: 12/08 |
| Origanum majorana | marjoram | leaves | Zielpunkt Warenhandel, Plus Warenhaus, 45466 Mühlheim; batch: MHD 2009 |
| Origanum vulgare | oregano | leaves | Ostmann Gewürze, D-33596 Bielefeld; batch: L7026CD |
| Pimpinella anisum | aniseed | seed | Ostmann Gewürze, D-33596 Bielefeld; batch: L6280AS |
| Plantago major | greater plantain | leaves | collection *, D-75223 Niefern; collection: 06.08.07 |
| Platanus | planes | bark | collection *, D-75223 Niefern; collection: 08.03.07 |
| Prunus cerasus | sour cherry | fruit | collection *, D-75223 Niefern; collection: 15.05.07 |
| Pyrus communis | pear williams' | peel | Fruit du monde, Plus Warenhaus, 45466 Mühlheim; batch: L 11/5 |
| Quercus | oak tree | leaves | collection *, D-75223 Niefern; collection: 05.06.07 |
| Rosmarinus officinalis | rosemary | leaves | Heinrich Klenk, D-97525 Schwebheim; batch: 2261 A 051201 03 |
| Salvia officinalis | sage | leaves | Ostmann Gewürze, D-33596 Bielefeld; batch: 6123AA |
| Sambucus nigra | black elder | leaves | collection *, D-75223 Niefern; collection: 06.08.07 |
| Sambucus nigra | black elder | bark | collection *, D-75223 Niefern; collection: 06.08.07 |
| Satureja montana | winter savory | leaves | collection *, D-75223 Niefern; collection: 06.08.07 |
| Solanum lycopersicum | tomato | peel | Rewe, D-75223 Niefern; batch: 26.03.07 |
| Syringa | lilac | leaves | collection *, D-75223 Niefern; collection: 13.05.07 |
| Syzygium aromaticum | clove | flower | Ostmann Gewürze, D-33596 Bielefeld; batch: L6326DB |
| Thymus vulgaris | common thyme | leaves | Ostmann Gewürze, D-33596 Bielefeld; batch: L6300DD |
| Verbena officinalis | common vervain | herb | Linden Apotheke, D-75223 Niefern; batch: 22.06.07 |
| Viscum album ssp. album | mistletoe (apple tree) | sprouts | collection *, D-75223 Niefern; collection: 12.07.07 |
| Vitis vinifera | grape vine | leaves | collection *, D-75223 Niefern; collection: 06.08.07 |
Plant material
Quantification of triterpenes within plant material
| Standard | Batch | Manufacturer |
|---|---|---|
| lupeol (LU) | 061K1772 | Sigma-Aldrich, Munich, Germany |
| betulin (BE) | BE 150307 | Carl Gustav Carus-Institute, Niefern, Germany |
| betulinic acid (BA) | 34255520 | Carl Roth, Karlsruhe, Germany |
| β-amyrin (bAM) | 0016 S 16 | Extrasynthese, Genay Cedex, France |
| erythrodiol (ER) | 114611124804002 | Fluka, Sigma-Aldrich, Munich, Germany |
| oleanolic acid (OA) | 38681978 | Carl Roth, Karlsruhe, Germany |
| α-amyrin (aAM) | 0015 S 06 | Extrasynthese, Genay Cedex, France |
| ursolic acid (UA) | 2208J | MP Biomedicals, Ilkirch, France |
| maslinic acid (MA) | 184071-200621 | Cayman Chemicals Ann Arbor, Michigan USA |
Preparation and characterization of triterpene dry extracts (TE)
Confirmation of triterpene identity

Statistics
Acknowledgements
References and Notes
- Allouche, Y.; Jimenez, A.; Uceda, M.; Aguilera, M.P.; Gaforio, J.J.; Beltran, G. Triterpenic content and chemometric analysis of virgin olive oils from forty olive cultivars. J. Agric. Food Chem. 2009, 57, 3604–3610. [Google Scholar] [CrossRef]
- Abe, F.; Yamauchi, T.; Nagao, T.; Kinjo, J.; Okabe, H.; Higo, H.; Akahane, H. Ursolic acid as a trypanocidal constituent in rosemary. Biol. Pharm. Bull. 2002, 25, 1485–1487. [Google Scholar] [CrossRef]
- Juan, M.E.; Wenzel, U.; Ruiz-Gutierrez, V.; Daniel, H.; Planas, J.M. Olive fruit extracts inhibit proliferation and induce apoptosis in HT-29 human colon cancer cells. J. Nutr. 2006, 136, 2553–2557. [Google Scholar]
- Horiuchi, K.; Shiota, S.; Hatano, T.; Yoshida, T.; Kuroda, T.; Tsuchiya, T. Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on vancomycin-resistant enterococci (VRE). Biol. Pharm. Bull. 2007, 30, 1147–1149. [Google Scholar] [CrossRef]
- Gu, J.Q.; Wang, Y.; Franzblau, S.G.; Montenegro, G.; Timmermann, B.N. Dereplication of pentacyclic triterpenoids in plants by GC-EI/MS. Phytochem. Anal. 2006, 17, 102–106. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Noshita, T.; Kidachi, Y.; Umetsu, H.; Hayashi, M.; Komiyama, K.; Funayama, S.; Royayama, K. Isolation of ursolic acid from apple peel and its specific efficacy as a potent antitumor agent. J. Health Sci. 2008, 54, 654–660. [Google Scholar] [CrossRef]
- He, X.; Liu, R.H. Triterpenoids isolated from apple peels have potent antiproliferative activity and may be partially responsible for apple's anticancer activity. J. Agric. Food. Chem. 2007, 55, 4366–4370. [Google Scholar] [CrossRef]
- Gotfredsen, E. Liber Herbarum II: The incomplete reference-guide to Herbal medicine. Available online: http://www.liberherbarum.com/ (27.03.2009).
- Chaturvedi, P.K.; Bhui, K.; Shukla, Y. Lupeol: connotations for chemoprevention. Cancer Lett. 2008, 263, 1–13. [Google Scholar] [CrossRef]
- Fulda, S. Betulinic acid for cancer treatment and prevention. Int. J. Mol. Sci. 2008, 9, 1096–1107. [Google Scholar] [CrossRef]
- Alakurtti, S.; Makela, T.; Koskimies, S.; Yli-Kauhaluoma, J. Pharmacological properties of the ubiquitous natural product betulin. Eur. J. Pharm. Sci. 2006, 29, 1–13. [Google Scholar] [CrossRef]
- Liu, J. Oleanolic acid and ursolic acid: research perspectives. J. Ethnopharmacol. 2005, 100, 92–94. [Google Scholar] [CrossRef]
- Jäger, S.; Laszczyk, M.N.; Scheffler, A. A preliminary pharmacokinetic study of betulin, the main pentacyclic triterpene from extract of outer bark of birch (Betulae alba cortex). Molecules 2008, 13, 3224–3235. [Google Scholar] [CrossRef]
- Dr., Duke, J. Duke's Phytochemical and Ethnobotanical Databases. Available online: http://www.ars-grin.gov/duke/plants.html (27.03.2009).
- Laszczyk, M.; Jäger, S.; Simon-Haarhaus, B.; Scheffler, A.; Schempp, C.M. Physical, chemical and pharmacological characterization of a new oleogel-forming triterpene extract from the outer bark of birch (betulae cortex). Planta Med. 2006, 72, 1389–1395. [Google Scholar] [CrossRef]
- Xi, J.; Chang, Q.; Chan, C.K.; Meng, Z.Y.; Wang, G.N.; Sun, J.B.; Wang, Y.T.; Tong, H.H.; Zheng, Y. Formulation development and bioavailability evaluation of a self-nanoemulsified drug delivery system of oleanolic acid. AAPS PharmSciTech 2009, 10, 172–182. [Google Scholar] [CrossRef]
- Huyke, C.; Laszczyk, M.; Scheffler, A.; Ernst, R.; Schempp, C.M. Treatment of actinic keratoses with birch bark extract: a pilot study. JDDG 2006, 4, 132–136. [Google Scholar] [CrossRef]
- Son, L.B.; Kaplun, A.P.; Symon, A.V.; Shpilevsky, A.A.; Grigoriev, V.B.; Shvets, V.I. Liposomal form of betulinic acid, a selective apoptosis inducing in melanoma cells substance. J. Liposome Res. 1998, 8, 78. [Google Scholar]
- Guo, M.; Zhang, S.; Song, F.; Wang, D.; Liu, Z.; Liu, S. Studies on the non-covalent complexes between oleanolic acid and cyclodextrins using electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2003, 38, 723–731. [Google Scholar] [CrossRef]
- Silva, M.G.; Vieira, I.G.; Mendes, F.N.; Albuquerque, I.L.; dos Santos, R.N.; Silva, F.O.; Morais, S.M. Variation of ursolic acid content in eight Ocimum species from northeastern Brazil. Molecules 2008, 13, 2482–2487. [Google Scholar]
- Razborsek, M.I.; Voncina, D.B.; Dolecek, V.; Voncina, E. Determination of oleanolic, betulinic and ursolic acid in Lamiaceae and mass spectral fragmentation of their trimethylsilylated derivatives. Chromatographia 2008, 67, 433–440. [Google Scholar] [CrossRef]
- Jäger, S.; Winkler, K.; Pfüller, U.; Scheffler, A. Solubility studies of oleanolic acid and betulinic acid in aqueous solutions and plant extracts of Viscum album L. Planta Med. 2007, 73, 157–162. [Google Scholar] [CrossRef]
- Sanchez-Avila, N.; Priego-Capote, F.; Ruiz-Jimenez, J.; de Castro, M.D. Fast and selective determination of triterpenic compounds in olive leaves by liquid chromatography-tandem mass spectrometry with multiple reaction monitoring after microwave-assisted extraction. Talanta 2009, 78, 40–48. [Google Scholar] [CrossRef]
- Jones, S. HPLC in a world without acetonitrile. Int. Labmate 2009, 34, 8–10. [Google Scholar]
- Kowalski, R. Studies of selected plant raw materials as alternative sources of triterpenes of oleanolic and ursolic acid types. J. Agric. Food Chem. 2007, 55, 656–662. [Google Scholar] [CrossRef]
- Ellgardt, K.; Bachelor, SLU, Alnarp. Triterpenes in apple cuticle of organically and IP cultivated apples. 2006. [Google Scholar]
- Frighetto, R.T.S.; Welendorf, R.M.; Nigro, E.N.; Frighetto, N. Isolation of ursolic acid from apple peels by high speed counter-current chromatography. Food Chem. 2008, 106, 767–771. [Google Scholar] [CrossRef]
- Davis, R.H.; DiDonato, J.J.; Johnson, R.W.; Stewart, C.B. Aloe vera, hydrocortisone, and sterol influence on wound tensile strength and anti-inflammation. J. Am. Podiatr. Med. Assoc. 1994, 84, 614–621. [Google Scholar]
- Akihisa, T.; Yasukawa, K.; Oinuma, H.; Kasahara, Y.; Yamanouchi, S.; Takido, M.; Kumaki, K.; Tamura, T. Triterpene alcohols from the flowers of compositae and their anti-inflammatory effects. Phytochemistry 1996, 43, 1255–1260. [Google Scholar] [CrossRef]
- Waller, G.R.; Jurzysta, M.; Karns, T.K.B.; Geno, P.W. Isolation and identification of ursolic acid from Coffea arabica L. (coffee) leaves. 14. In Association Scientifique Internationale pour le Café (ASIC) Conferences, San Francisco, USA, 1991.
- Jayaprakasam, B.; Olson, L.K.; Schutzki, R.E.; Tai, M.H.; Nair, M.G. Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in Cornelian cherry (Cornus mas). J. Agric. Food Chem. 2006, 54, 243–248. [Google Scholar] [CrossRef]
- Lin, Y.; Vermeer, M.A.; Trautwein, E.A. Triterpenic acids present in hawthorn lower plasma cholesterol by inhibiting intestinal ACAT activity in hamsters. Evid. Based Complement. Alternat. Med. 2009, 1–9. [Google Scholar]
- Siddiqui, B.S.; Sultana, I.; Begum, S. Triterpenoidal constituents from Eucalyptus camaldulensis var. obtusa leaves. Phytochemistry 2000, 54, 861–865. [Google Scholar] [CrossRef]
- Awad, R.; Muhammad, A.; Durst, T.; Trudeau, V.L.; Arnason, J.T. Bioassay-guided fractionation of lemon balm (Melissa officinalis L.) using an in vitro measure of GABA transaminase activity. Phytother. Res. 2009, 1–7, [Epub ahead of print]. [Google Scholar]
- Fu, L.; Zhang, S.; Li, N.; Wang, J.; Zhao, M.; Sakai, J.; Hasegawa, T.; Mitsui, T.; Kataoka, T.; Oka, S.; Kiuchi, M.; Hirose, K.; Ando, M. Three new triterpenes from Nerium oleander and biological activity of the isolated compounds. J. Nat. Prod. 2005, 68, 198–206. [Google Scholar] [CrossRef]
- Siddiqui, B.S.; Aslam, H.; Ali, S.T.; Begum, S.; Khatoon, N. Two new triterpenoids and a steroidal glycoside from the aerial parts of Ocimum basilicum. Chem. Pharm. Bull. (Tokyo) 2007, 55, 516–519. [Google Scholar] [CrossRef]
- Stiti, N.; Triki, S.; Hartmann, M.A. Formation of triterpenoids throughout Olea europaea fruit ontogeny. Lipids 2007, 42, 55–67. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, R.; Herrera, M.D.; de Sotomayor, M.A.; Ruiz-Gutierrez, V. Pomace olive oil improves endothelial function in spontaneously hypertensive rats by increasing endothelial nitric oxide synthase expression. Am. J. Hypertens. 2007, 20, 728–734. [Google Scholar] [CrossRef]
- Vagi, E.; Simandi, B.; Daood, H.G.; Deak, A.; Sawinsky, J. Recovery of pigments from Origanum majorana L. by extraction with supercritical carbon dioxide. J. Agric. Food Chem. 2002, 50, 2297–2301. [Google Scholar] [CrossRef]
- Chiang, L.C.; Ng, L.T.; Chiang, W.; Chang, M.Y.; Lin, C.C. Immunomodulatory activities of flavonoids, monoterpenoids, triterpenoids, iridoid glycosides and phenolic compounds of Plantago species. Planta Med. 2003, 69, 600–604. [Google Scholar] [CrossRef]
- Galgon, T.; Hoke, D.; Drager, B. Identification and quantification of betulinic acid. Phytochem. Anal. 1999, 10, 187–190. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef]
- Liao, Q.F.; Xie, S.P.; Chen, X.H.; Bi, K.S. Study on the chemical constituents of Sambucus chinensis Lindl. Zhong Yao Cai 2006, 29, 916–918. [Google Scholar]
- Mintz-Oron, S.; Mandel, T.; Rogachev, I.; Feldberg, L.; Lotan, O.; Yativ, M.; Wang, Z.; Jetter, R.; Venger, I.; Adato, A.; Aharoni, A. Gene expression and metabolism in tomato fruit surface tissues. Plant Physiol. 2008, 147, 823–851. [Google Scholar] [CrossRef]
- Cai, L.; Wu, C.D. Compounds from Syzygium aromaticum possessing growth inhibitory activity against oral pathogens. J. Nat. Prod. 1996, 59, 987–990. [Google Scholar] [CrossRef]
- Rowe, E.J.; Orr, J.E.; Uhl, A.H.; Parks, L.M. Isolation of oleanolic acid and ursolic acid from Thymus vulgaris. L. J. Am. Pharm. Assoc. Am. Pharm. Assoc. 1949, 38, 122–124. [Google Scholar]
- Deepak, M.; Handa, S.S. Antiinflammatory activity and chemical composition of extracts of Verbena officinalis. Phytother. Res. 2000, 14, 463–465. [Google Scholar] [CrossRef]
- Eiznhamer, D.A.; Xu, Z.Q. Betulinic acid: a promising anticancer candidate. IDrugs 2004, 7, 359–373. [Google Scholar]
- Scher, J.; Urech, K.; Becker, H. Triterpene in der Mistel Viscum album L. Mistilteinn 2006, 7, 16–29. [Google Scholar]
- Guinda, A. Use of solid residue from the olive industry. Grassasy Aceites 2006, 57, 107–115. [Google Scholar]
- van Beek, T.A.; Montoro, P. Chemical analysis and quality control of Ginkgo biloba leaves, extracts, and phytopharmaceuticals. J. Chromatogr. A 2009, 1216, 2002–2032. [Google Scholar]
- Sample Availability: Samples of several triterpene dry extracts (TE) are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants – Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules 2009, 14, 2016-2031. https://doi.org/10.3390/molecules14062016
Jäger S, Trojan H, Kopp T, Laszczyk MN, Scheffler A. Pentacyclic Triterpene Distribution in Various Plants – Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules. 2009; 14(6):2016-2031. https://doi.org/10.3390/molecules14062016
Chicago/Turabian StyleJäger, Sebastian, Holger Trojan, Thomas Kopp, Melanie N. Laszczyk, and Armin Scheffler. 2009. "Pentacyclic Triterpene Distribution in Various Plants – Rich Sources for a New Group of Multi-Potent Plant Extracts" Molecules 14, no. 6: 2016-2031. https://doi.org/10.3390/molecules14062016
APA StyleJäger, S., Trojan, H., Kopp, T., Laszczyk, M. N., & Scheffler, A. (2009). Pentacyclic Triterpene Distribution in Various Plants – Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules, 14(6), 2016-2031. https://doi.org/10.3390/molecules14062016