Synthesis of a Diamino Substituted Terphenyldivinyl Chromophore
Abstract
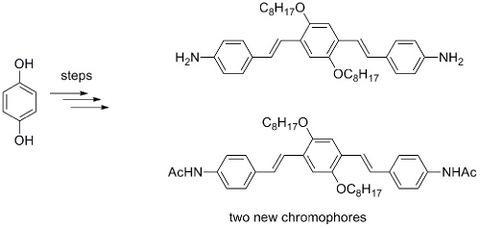
:Introduction

Results and Discussion



Experimental
General
Conclusions
Acknowledgements
References and Notes
- Baik, C.; Kim, D.; Kang, M.S.; Kang, S.O.; Ko, J.; Nazeeruddin, M.K.; Gratzel, M. Organic dyes with a novel anchoring group for dye-sensitized solar cell applications. J. Photochem. Photobiol. A 2009, 201, 168–174. [Google Scholar] [CrossRef]
- Yum, J.H.; Hagberg, D.P.; Moon, S.J.; Karlsson, K.M.; Marinado, T.; Sun, L.C.; Hagfeldt, A.; Nazeeruddin, M.K.; Gratzel, M. A Light-Resistant Organic Sensitizer for Solar-Cell Applications. Angew. Chem. Int. Edit. 2009, 48, 1576–1580. [Google Scholar]
- Yum, J.H.; Jung, I.; Baik, C.; Ko, J.; Nazeeruddin, M.K.; Gratzel, M. High efficient donor-acceptor ruthenium complex for dye-sensitized solar cell applications. Energy Environ. Sci. 2009, 2, 100–102. [Google Scholar] [CrossRef]
- Tang, C.W.; Vanslyke, S.A. Organic electroluminescent diodes. J. Appl. Phys. 1987, 51, 913–915. [Google Scholar]
- Mason, M.G.; Hung, L.S.; Tang, C.W.; Lee, S.T.; Wong, K.W.; Wang, M. Characterization of treated indium-tin-oxide surfaces used in electroluminescent devices. J. Appl. Phys. 1999, 86, 1688–1692. [Google Scholar]
- Jin, J.Y.; Jin, Z.Z.; Xia, Y.; Zhou, Z.Y.; Wu, X.; Zhu, D.X.; Su, Z.M. Design and synthesis of 1,4-bis[4-(1,1-dicyanovinyl)styryl]-2,5-bis(alkoxy)benzenes as red organic electroluminescent PPV analogs. Polymer 2007, 48, 4028–4033. [Google Scholar] [CrossRef]
- Cacialli, F.; Feast, W.J.; Friend, R.H.; de Jong, M.; Lovenich, P.W.; Salaneck, W.R. Synthesis and characterisation of poly(distyrylbenzene-block-hexa(ethylene oxide)) and its fluorinated analogue-two new block copolymers and their application in electroluminescent devices. Polymer 2002, 43, 3555–3561. [Google Scholar] [CrossRef]
- Kimoto, A.; Masachika, K.; Cho, J.S.; Higuchi, M.; Yamamoto, K. Synthesis and electroluminescence properties of novel main chain poly(p-phenylenevinylene)s possessing pendant phenylazomethine dendrons as metal ligation sites. Chem. Mater. 2004, 16, 5706–5712. [Google Scholar] [CrossRef]
- Kimura, M.; Sato, M.; Adachi, N.; Fukawa, T.; Kanbe, E.; Shirai, H. Poly(p-phenylene vinylene)s wrapped with 1,3,5-phenylene-based rigid dendrons. Chem. Mater. 2007, 19, 2809–2815. [Google Scholar] [CrossRef]
- Wu, C.Y.; Tian, D.H.; Feng, Y.; Polak, P.; Wei, J.J.; Sharp, A.; Stankoff, B.; Lubetzki, C.; Zalc, B.; Mufson, E.J.; Gould, R.M.; Feinstein, D.L.; Wang, Y.M. A novel fluorescent probe that is brain permeable and selectively binds to myelin. J. Histochem. Cytochem. 2006, 54, 997–1004. [Google Scholar] [CrossRef]
- Abbel, R.; Grenier, C.; Pouderoijen, M.J.; Stouwdam, J.W.; Leclere, P.; Sijbesma, R.P.; Meijer, E.W.; Schenning, A. White-Light Emitting Hydrogen-Bonded Supramolecular Copolymers Based on pi-Conjugated Oligomers. J. Am. Chem. Soc. 2009, 131, 833–843. [Google Scholar] [CrossRef]
- Shanks, D.; Preus, S.; Qvortrup, K.; Hassenkam, T.; Nielsen, M.B.; Kilsa, K. Excitation energy transfer in novel acetylenic perylene diimide scaffolds. New J. Chem. 2009, 33, 507–516. [Google Scholar] [CrossRef]
- Takahashi, M.; Ichihashi, Y.; Nishizawa, N.; Ohno, S.; Fujit, N.; Yamashita, M.; Sengoku, T.; Yoda, H. Construction of light-harvesting reverse micelles in nanoscopic dimensions. J. Photoch. Photobio. A 2009, 203, 56–63. [Google Scholar] [CrossRef]
- Wang, H.Y.; Pu, K.Y.; Huang, S.; Liu, F.; Peng, B.; Wei, W. Alternating copolymers based on perylene bisimide and oligo(p-phenylene ethynylene) units: Synthesis, characterization, and photoinduced energy and electron transfer processes of a new class of donor-acceptor systems. React. Funct. Polym. 2009, 69, 117–123. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, C.H.; Li, Y.J.; Li, Y.L.; Wang, S.; Zhuang, J.P.; Liu, H.B.; Wang, N.; He, X.R.; Li, Y.F.; Zhu, D.B. Synthesis and photovoltaic characteristics of novel copolymers containing poly(phenylenevinylene) and triphenylamine moieties connected at 1,7 bay positions of perylene bisimide. Macromolecules 2005, 38, 716–721. [Google Scholar] [CrossRef]
- Syamakumari, A.; Schenning, A.P.H.J.; Meijer, E.W. Synthesis, optical properties, and aggregation behavior of a triad system based on perylene and oligo(p-phenylene vinylene) units. Chem. Eur. J. 2002, 9, 3353–3361. [Google Scholar] [CrossRef]
- Bellamy, F.D.; Ou, K. Selective reduction of aromatic nitro compounds with stannous chloride in non acidic and non aqueous medium. Tetrahedron Lett. 1984, 25, 839–842. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1 to 7 are available from the authors.
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Du, Z.-T.; Liu, R.; Wang, J.-R.; Li, A.-P. Synthesis of a Diamino Substituted Terphenyldivinyl Chromophore. Molecules 2009, 14, 2111-2117. https://doi.org/10.3390/molecules14062111
Du Z-T, Liu R, Wang J-R, Li A-P. Synthesis of a Diamino Substituted Terphenyldivinyl Chromophore. Molecules. 2009; 14(6):2111-2117. https://doi.org/10.3390/molecules14062111
Chicago/Turabian StyleDu, Zhen-Ting, Ru Liu, Jun-Ru Wang, and An-Pai Li. 2009. "Synthesis of a Diamino Substituted Terphenyldivinyl Chromophore" Molecules 14, no. 6: 2111-2117. https://doi.org/10.3390/molecules14062111
APA StyleDu, Z. -T., Liu, R., Wang, J. -R., & Li, A. -P. (2009). Synthesis of a Diamino Substituted Terphenyldivinyl Chromophore. Molecules, 14(6), 2111-2117. https://doi.org/10.3390/molecules14062111