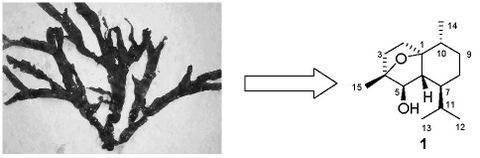
A New Cadinane Sesquiterpene from the Marine Brown Alga Dictyopteris divaricata
Abstract
:Introduction
Results and Discussion
Experimental
General
Algal Material
Extraction and Isolation
Brine Shrimp Assays
Acknowledgements
References and Notes
- Song, F.; Xu, X.; Li, S.; Wang, S.; Zhao, J.; Yang, Y.; Fan, X.; Shi, J.; He, L. Minor sesquiterpenes with new carbon skeleton from the brown alga Dictyopteris divaricata. J. Nat. Prod. 2006, 69, 1261–1266. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Xu, X.; Li, S.; Wang, S.; Zhao, J.; Cao, P.; Yang, Y.; Fan, X.; Shi, J.; He, L.; Lü, Y. Norsesquiterpenes from the brown alga Dictyopteris divaricata. J. Nat. Prod. 2005, 68, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Fan, X.; Xu, X.; Zhao, J.; Yang, Y.; Shi, J. Cadinane sesquiterpenes from the brown alga Dictyopteris divaricata. J. Nat. Prod. 2004, 67, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- König, G.M.; Wright, A.D. Concerted application of a shift reagent and 2D NOESY to the structure determination of new natural products from the tropical brown alga Dictyopteris delicatula. Magn. Reson. Chem. 1995, 33, 178–183. [Google Scholar] [CrossRef]
- Segawa, M.; Yamano, K.; Shrahama, H. A germacrane-type sesquiterpene from the brown alga Dictyopteris divaricata. Phytochemistry 1990, 29, 973–974. [Google Scholar] [CrossRef]
- Suzuki, M.; Kowata, N.; Kurosawa, E.; Kobayashi, H.; Tanaka, I. The structure of a germacrane-tpye sesquiterpene alcohol, a possible precursor of guaiane-type sesquiterpenes from the brown alga Dictyopteris divaricata. Chem. Lett. 1990, 19, 2187–2190. [Google Scholar] [CrossRef]
- Kajiwara, T.; Hatanaka, A.; Tanaka, Y.; Kawai, T.; Ishihara, M.; Tsuneya, T.; Fujimura, T. Volatile constituents from marine brown algae of Japanese Dictyopteris. Phytochemistry 1989, 28, 636–639. [Google Scholar] [CrossRef]
- Suzuki, M.; Kowata, N.; Kurosawa, E. Epicubebol and related sesquiterpenoids from the brown alga Dictyopteris divaricata. Bull. Chem. Soc. Jpn. 1981, 54, 2366–2368. [Google Scholar] [CrossRef]
- Chyu, C.F.; Ke, M.R.; Chang, Y.S.; Chien, S.C.; Kuo, Y.H. New cadinane-type sesquiterpenes from the roots of Taiwania cryptomerioides HAYATA. Helv. Chim. Acta 2007, 90, 1514–1521. [Google Scholar] [CrossRef]
- Solis, P.N.; Wright, C.W.; Anderson, M.M.; Gupta, M.P.; Phillipson, J.D. A microwell cytotoxicity assay using Artemia salina (Brine shrimp). Planta Med. 1993, 59, 250–252. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of compound 1 are available from the authors. |

| No. | δC | δH | 1H-1H COSY |
|---|---|---|---|
| 1 | 87.1 s | ||
| 2a | 34.6 t | 1.42 (ddd, 12.5, 9.6, 5.8) | H-2b, H-3a, H-3b |
| 2b | 1.94 (ddd, 12.5, 12.1, 4.0) | H-2a, H-3a, H-3b | |
| 3a | 29.4 t | 1.30 (ddd, 12.1, 11.9, 5.8) | H-3b, H-2a, H-2b |
| 3b | 2.21 (ddd, 11.9, 9.6, 4.0) | H-3a, H-2a, H-2b | |
| 4 | 85.7 s | ||
| 5 | 85.5 d | 3.46 (br s) | H-6 |
| 6 | 56.0 d | 1.26 (d, 11.6) | H-5, H-7 |
| 7 | 47.3 d | 1.14 (dddd, 12.1, 11.6, 2.1, 1.6) | H-6, H-8a, H-8b |
| 8a | 23.7 t | 0.89 (dddd, 12.9, 12.6, 12.1, 2.1) | H-7, H-8b, H-9a, H-9b |
| 8b | 1.54 (br dd, 12.9, 3.1) | H-7, H-8a, H-9a, H-9b | |
| 9a | 31.7 t | 1.23 (m) | H-8a, H-8b, H-9b, H-10 |
| 9b | 1.62 (m) | H-8a, H-8b, H-9a, H-10 | |
| 10 | 34.9 d | 1.59 (m) | H-9a, H-9b, H-14 |
| 11 | 27.3 d | 1.80 (hept d, 6.9, 1.6) | H-7, H-12, H-13 |
| 12 | 16.0 q | 0.80 (d, 6.9) | H-11 |
| 13 | 21.8 q | 0.94 (d, 6.9) | H-11 |
| 14 | 15.3 q | 1.01 (d, 6.5) | H-10 |
| 15 | 19.6 q | 1.41 (s) |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wen, W.; Li, F.; Ji, N.-Y.; Li, X.-M.; Cui, C.-M.; Li, X.-D.; Zhang, L.-N.; Xue, Q.-Z.; Wang, B.-G. A New Cadinane Sesquiterpene from the Marine Brown Alga Dictyopteris divaricata. Molecules 2009, 14, 2273-2277. https://doi.org/10.3390/molecules14062273
Wen W, Li F, Ji N-Y, Li X-M, Cui C-M, Li X-D, Zhang L-N, Xue Q-Z, Wang B-G. A New Cadinane Sesquiterpene from the Marine Brown Alga Dictyopteris divaricata. Molecules. 2009; 14(6):2273-2277. https://doi.org/10.3390/molecules14062273
Chicago/Turabian StyleWen, Wei, Fang Li, Nai-Yun Ji, Xiao-Ming Li, Chuan-Ming Cui, Xiao-Dong Li, Li-Na Zhang, Qin-Zhao Xue, and Bin-Gui Wang. 2009. "A New Cadinane Sesquiterpene from the Marine Brown Alga Dictyopteris divaricata" Molecules 14, no. 6: 2273-2277. https://doi.org/10.3390/molecules14062273
APA StyleWen, W., Li, F., Ji, N. -Y., Li, X. -M., Cui, C. -M., Li, X. -D., Zhang, L. -N., Xue, Q. -Z., & Wang, B. -G. (2009). A New Cadinane Sesquiterpene from the Marine Brown Alga Dictyopteris divaricata. Molecules, 14(6), 2273-2277. https://doi.org/10.3390/molecules14062273