Synthesis of Symmetrical and Non-symmetrical Diimines from Dimedone
Abstract
:Introduction
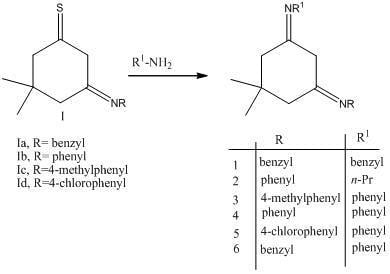
Results and Discussion



| Method | B3LYP/6-31G(d,p) | B3LYP/6-31G(d) | B3WP91/6-31G(d) | HF/6-31G(d) | |
|---|---|---|---|---|---|
| Tautomer | |||||
| II | –687.530 | -696.301 | -696.242 | -692.427 | |
| III | –696.631 | -696.310 | -696.249 | -692.435 | |
| IV | –696.775 | -687.395 | -688.313 | -684.111 | |
| B3LYP | B3WP91 | HF | Exp. | Assignment |
|---|---|---|---|---|
| 3481 | 3498 | 3470 | 3200 | υ(NH) |
| 3094 | 3088 | 3055 | 3117 | υ(CH)C=C-H |
| 3081 | 3079 | 3030 | 3090 | υ(CH)phenyl |
| 3022 | 3025 | 2955 | 2991 | υ(CH) |
| 3004 | 3005 | 2940 | 2966 | υ(CH) |
| 2965 | 2964 | 2922 | 2958 | υ(CH) |
| 2926 | 2930 | 2917 | 2925 | υ(CH) |
| 2920 | 2921 | 2890 | 2891 | υ(CH) |
| 2900 | 2901 | 2851 | 2808 | υ(CH) |
| 1616 | 1924 | 1693 | 1600 | υ(C=N) |
| 1605 | 1609 | 1618 | 1593 | υ(C=C)+ (C=N) |
| 1561 | 1566 | 1577 | 1560 | υ(C=C) |
| 1503 | 1502 | 1518 | 1508 | δ(N-H) |
| 1478 | 1470 | 1486 | 1466 | δ(Me) |
| 1432 | 1421 | 1443 | 1447 | δ(CH2) |
| 1426 | 1414 | 1441 | 1410 | ω(Me)N-Me |
| 1392 | 1381 | 1411 | 1381 | ω (Me)C-Me |
| 1351 | 1346 | 1336 | 1368 | δ(CH2) |
| 1321 | 1322 | 1330 | 1297 | δ(C-H)C=C-H |
| 1241 | 1256 | 1256 | 1241 | υ(CH2-C-CH2) asym. |
| 1037 | 1047 | 1032 | 1027 | υ(Me)N-Me |
| 1011 | 1000 | 1017 | 1005 | Ph breath |
| 974 | 969 | 972 | 975 | Ph breath |
| 940 | 938 | 916 | 926 | δring |
| 895 | 902 | 901 | 896 | υ(CH2-C-CH2)sym |
| 851 | 857 | 853 | 840 | υ(C-C ) |
| 811 | 810 | 811 | 787 | δring |
| 683 | 681 | 700 | 705 | δring |

Experimental
General
General method for the preparation of diimines
Computational method
Conclusions
References
- Bourget-Marle, M.; Lappert, M.F.; Seven, J.R. The Chemistry of β–Diketiminatometal Complexes. Chem. Rev. 2002, 102, 3031–3065. [Google Scholar] [CrossRef]
- Barluenga, J.; Olano, B.; Fustero, S. Reduction of 1,3-Diimines. A New and General Method of Synthesis of γ-Diamines, β-Amino Ketones and Derivatives withTwo and Three Chiral Centers. J. Org. Chem. 1983, 20, 2255–2259. [Google Scholar] [CrossRef]
- Armesto, D.; Bosch, P.; Gallego, M.G.; Martin, J.F.; Ortiz, M.J.; Perez-Ossorio, R.; Ramos, A. Synthesis of Diimines from 1,2-Dicarbonyl Compounds by direct Catalized Condensation. Org. Prep. Proced. Int. 1987, 19, 181–186. [Google Scholar] [CrossRef]
- Lee, Y.W.; Kim, Y.; Sim, W.; Park, C.H.; Ahn, Y.M. Reactions of Aryl Organometallic Reagents with Isomers of Phthalonitriles: Triaryl Diketimines and Diketones. Bull. Korean Chem. Soc. 1986, 7, 362–366. [Google Scholar]
- Qian, B.; Ward, D.L.; Smith, M.R. Synthesis, Structure, and Reactivity of β-Diketiminato Aluminum Complexes. Organometallics 1998, 17, 3070–3076. [Google Scholar] [CrossRef]
- Abood, N.A.; Aiube, Z.H.; Saeed, B.A. Spectroscopic Study of New Schiff Bases Derived from Dibenzoylmethane and Benzoylacetone. J. Chem. Soc. Pak. 1992, 14, 97–101. [Google Scholar]
- Dudek, G.O.; Dudek, P. Spectroscopic Studies of Keto-Enol Equilibria. VII. Nitrogen-15 Substituted Schiff Bases. J. Am. Chem. Soc. 1964, 20, 4283–4285. [Google Scholar] [CrossRef]
- Zheglova, D.K.; Genova, D.G.; Bolvig, S.; Hansen, P.E. Deuterium Isotope Effectes on 13C Chemical Shifts of Enaminones. Acta Chem. Scand. 1997, 51, 1016–1023. [Google Scholar] [CrossRef]
- Eberlin, M.N.; Takahat, Y.; Kascheres, C. The Use of AM1 in Structural Analyses of Primary and Secondary Enaminones. Theochem. 1990, 207, 143–156. [Google Scholar] [CrossRef]
- Kascheres, C.M. The Chemistry of Enaminones, Diazocarbonyls and Small Rings: Our Contribution. J. Braz. Chem. Soc. 2003, 14, 945–969. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density-functional theory of atoms and molecules; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Dixit, N.; Reddy, K.V.; Deshmukh, A.S.; Rajappa, S. Conformational Preferences of α-Functionalised Keten-S, N-acetals: Potential role of S..O and S..S Interaction in Solution. Tetrahedron 1995, 51, 1437–1448. [Google Scholar] [CrossRef]
- Granovsky, A. PC GAMESS/Firefly version 7.1.F. http://classic.chem.msu.su/gran/gamess/index.html.
Sample Availability: Samples of the compounds are available from the authors. |
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Saeed, B.A.; Musad, I.A. Synthesis of Symmetrical and Non-symmetrical Diimines from Dimedone. Molecules 2009, 14, 2278-2285. https://doi.org/10.3390/molecules14062278
Saeed BA, Musad IA. Synthesis of Symmetrical and Non-symmetrical Diimines from Dimedone. Molecules. 2009; 14(6):2278-2285. https://doi.org/10.3390/molecules14062278
Chicago/Turabian StyleSaeed, Bahjat A., and Ibrahim A. Musad. 2009. "Synthesis of Symmetrical and Non-symmetrical Diimines from Dimedone" Molecules 14, no. 6: 2278-2285. https://doi.org/10.3390/molecules14062278
APA StyleSaeed, B. A., & Musad, I. A. (2009). Synthesis of Symmetrical and Non-symmetrical Diimines from Dimedone. Molecules, 14(6), 2278-2285. https://doi.org/10.3390/molecules14062278