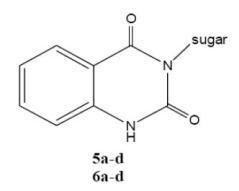
3.2. Preparation of 3-N-sugar-substituted-2,4-(1H,3H)-quinazolinediones
3.2.1. Synthesis and Spectral Data of 2a-c
Aminosugar 1a-c (2.9 g, 15 mmol), o-nitrobenzoic acid (3.2 g, 16.5 mmol) and 1-hydroxy benzotriazole (HOBt) (4.9 g, 36.3 mmol) were dissolved in DMF (80 mL). The mixture was cooled to 0 °C and stirred for 30 min. Then a solution of dicyclohexylcarbodiimide (DCC, 3.8 g, 18.2 mmol) in DMF (15 mL) was added dropwise. The mixture was stirred for 18 h at room temperature and filtered. The filtrate was evaporated to dryness under reduced pressure, and the residue was purified by column chromatography on silica gel to give 2a-c.
Methyl-6-(o-nitro)benzamidyl-6-deoxy-α-D-glucopyranoside (2a). White flocculant crystals; Yield: 47.0%; mp: 230-233 °C; [a]D +116o (c 1.01, MeOH); 1H-NMR δ (ppm) (DMSO-d6): 3.18 (m, 1H, H-6b), 3.34 (m, 1H, H-2), 3.38 (s, 3H, OCH3), 3.47 (m, 2H, H-4,5), 3.61 (m, 1H, H-3), 3.85 (m, 1H, H-5), 4.51 (d, 1H, J1, 2 1.2Hz, H-1), 4.67 (d, 1H, J 6.0 Hz, H-OH), 4.79 (d, 1H, J 5.1 Hz, H-OH), 4.94 (d, 1H, J 5.4 Hz, H-OH), 7.55-8.03 (m, 4H, Ph), 8.76 (t, 1H, J5.1Hz, H-NH); 13C-NMR δ (ppm) (DMSO-d6): 40.8 (C-6), 54.4 (OCH3), 70.3 (C-5), 71.9 (C-3), 72.3 (C-2), 73.0 (C-4), 99.7 (C-1), 124.0 (Ph), 129.1 (Ph), 130.6 (Ph), 132.6 (Ph), 133.5 (Ph) , 147.1 (Ph), 165.1 (C=O); ESI-TOF-MS: [M+1]+ m/z 343.0; [M+Na]+ m/z 365.0.
Methyl-6-(o-nitro)benzamidyl-6-deoxy-α-D-galactopyranoside (2b). White flocculant crystals; Yield: 42.3%; mp: 218- 220 °C; [a]D +52o (c 1.01, DMSO); 1H-NMR δ (ppm) (DMSO-d6): 3.28 (s, 3H, OCH3), 3.01-3.77 (m, 5H, sugar-H), 4.57 (m, 3H, sugar-H), 7.57-8.03 (m, 4H, Ph), 8.82 (d, 1H, H-NH); 13C-NMR δ (ppm) (DMSO-d6): 45.5 (C-6), 54.6 (OCH3), 68.3 (C-2), 68.4 (C-5), 69.3 (C-4), 69.4 (C-3), 100.2 (C-1), 124.0 (Ph), 129.0 (Ph), 130.7 (Ph), 132.4 (Ph), 133.5 (Ph), 147.1 (Ph), 165.7 (C=O); ESI-TOF-MS: [M+1]+ m/z 343.0; [M+Na]+ m/z 365.0.
Methyl-6-(o-nitro)benzamidyl-6-deoxy-α-D-mannopyranoside (2c). White flocculant crystals; Yield: 47.0%; mp: 188-189 °C; [a]D +56o (c 1.01, CHCl3); 1H-NMR δ (ppm) (DMSO-d6): 3.13-3.49 (m, 5H, H-sugar), 3.39 (s, 3H, OCH3), 4.60 (m, 1H, H-sugar), 4.50 (s, 1H, H-1), 4.77 (d, J 8.7 Hz, H-OH), 4.78(d, J 4.8 Hz, H-OH), 4.92 (d, J 5.4 Hz, H-OH), 7.54-8.03 (m, 4H, Ph), 8.76 (t, 1H, J 5.1 Hz, H-NH); 13C-NMR δ (ppm) (DMSO-d6): 41.0 (C-6), 54.1 (OCH3), 69.6 (C-4), 70.2 (C-5), 70.6 (C-3), 71.4 (C-2), 101.0 (C-1), 124.0 (Ph), 129.1 (Ph), 130.6 (Ph), 132.6 (Ph), 133.5 (Ph), 147.1 (Ph), 165.9 (C=O); ESI-TOF-MS: [M+1]+ m/z 343.0; [M+Na]+ m/z 365.0.
3.2.2. Synthesis and Spectral Data of 2-(o-nitro)benzamidyl-2-deoxy-β-D-glucopyranose (2d)
Glucosamine hydrochloride (7.8 g, 36 mmol) and sodium methoxide (2.25 g, 41.7 mmol) were added to methanol (100 mL). The mixture was stirred for 20 min and then evaporated to dryness under vacuum. The residue was dissolved in DMF (200 mL), followed by the addition of o-nitrobenzoic acid (5.1 g, 30 mmol) and 1-hydroxybenzotriazole (HOBt, 9.5 g, 72 mmol). The mixture was cooled to 0 °C and stirred for 30 min. Then the solution of dicyclohexylcarbodiimide (DCC, 6.9 g, 36 mmol) in DMF (25 mL) was added dropwise. The mixture was stirred for 20 h at room temperature and filtered. The filtrate was evaporated to dryness under reduced pressure, and the residue was purified through column chromatography on silica gel to yield 5.5 g of white flocculant crystals of 2d; yield: 47%; mp: 208-212 °C; [a]D +40o (c 1.01, MeOH); 1H-NMR δ (ppm) (DMSO-d6): 3.28 (m, 1 H, H-sugar), 3.43-3.83 (m, 5 H, H-sugar), 4.55 (t, 1H, J 5.7 Hz, H-OH), 4.80 (d, 1H, J5.4Hz, H-OH), 5.05 (d, 1H, J 5.4 Hz, H-OH),5.18 (s, 1H, H-1), 6.62 (d, 1H, J 4.2 Hz H-OH), 7.73-8.10 (m, 4H, Ph), 8.64(d, 1H, J 8.1 Hz, H-NH); 13C-NMR δ(ppm) (75MHz, DMSO-d6): 55.1, 61.1, 70.1, 71.1, 72.1 (C of sugar ring), 90.4 (C-1), 123.8 (Ph), 129.5 (Ph), 130.6 (Ph), 132.4 (Ph), 133.1 (Ph), 147.3(Ph), 165.5(C=O); ESI-TOF-MS: [M+1]+ m/z 329.0; [M+Na]+ m/z 351.0.
3.2.3. Synthesis and Spectral Data of 3a-d
The appropriate 2-nitro-(N-sugar-substituted) benzamide 2a-d (2.0 g) was dissolved in pyridine (50 mL), followed by the addition of acetic anhydride (25 mL). The solution was stirred at room temperature overnight and evaporated to dryness under reduced pressure. The residue was dissolved in ethyl acetate and washed sequentially with saturated sodium hydrogen carbonate solution, saturated brine and water. The organic layer was dried over anhydrous Na2SO4 and evaporated under reduced pressure to give 3a-d as yellow solids.
Methyl-6-(o-nitro-)benzamidyl-6-deoxy-2,3,4-tri-O-acetyl-α-D-glucopyranoside (3a). Yield: 95.0 %; mp: 77-79 oC; [a]D +104 o (c 1.01, CHCl3); 1H-NMR δ (ppm) (CDCl3): 2.02 (s, 3H, Ac), 2.08 (s, 3H, Ac), 2.10 (s, 3H, Ac), 3.42 (s, 3H, OCH3), 3.61 (m, 1H, H-6), 3.79 (m, 1H, H-5), 4.04 (m, 1H, H-5), 4.83 (dd, 1H, J1,2 3.6 Hz, J2,3 10.2 Hz, H-2), 4.91 (d, 1H, J1, 2 3.6 Hz, H-1), 5.02 (t, 1H, J 9.9 Hz, H-3), 5.48 (t, 1H, J 9.9 Hz, H-4), 6.26 (t, 1H, J 5.7 Hz, H-NH), 7.27-8.09 (m, 4H, Ph); 13C-NMR δ (ppm) (CDCl3): 20.7 (CH3CO), 39.4 (C-6), 55.0 (OCH3), 67.6 (C-5), 69.2 (C-3), 69.8 (C-2), 70.9 (C-4), 96.8 (C-1), 124.5 (Ph), 129.0 (Ph), 130.5 (Ph), 132.7 (Ph), 133.9 (Ph), 146.2 (Ph), 166.6 (C=O), 169.9 (CH3CO), 170.2 (CH3CO), 170.3 (CH3CO); ESI-TOF-MS: [M+1]+ m/z 469.1; [M+Na]+ m/z 491.1.
Methyl-6-(o-nitro-)benzamidyl-6-deoxy-2,3,4-tri-O-acetyl-α-D-galactopyranoside (3b). Yield: 98.0%; mp: 87-90 °C; [a]D+68o (c 1.01, CHCl3); 1H-NMR δ (ppm) (CDCl3): 1.99 (s, 3H, Ac), 2.07 (s, 3H, Ac), 2.18 (s, 3H, Ac), 3.42(s, 3H, OCH3), 3.54 (m, 2H, H-6), 4.22 (t, 1H, J 6.6 Hz, H-5), 4.98 (d, 1H, J1, 2 3.0 Hz, H-1), 5.16 (dd, 1H, J1,2 3.0 Hz, J2,3 10.8 Hz, H-2), 5.37-5.46 (m, 2H, H-4, H-3), 6.42 (br, 1H, H-NH), 7.38-8.06 (m, 4H, Ph); 13C-NMR δ (ppm) (CDCl3): 20.6 (CH3CO), 20.7 (CH3CO), 20.8 (CH3CO), 39.3 (C-6), 55.7 (OCH3), 66.5 (C-5), 67.4 (C-3), 68.2 (C-2), 69.0 (C-4), 97.2 (C-1), 124.5 (Ph), 128.6 (Ph), 130.6 (Ph), 132.5 (Ph), 133.8 (Ph), 146.4 (Ph), 166.6 (C=O), 169.7 (CH3CO), 170.4 (CH3CO), 170.8 (CH3CO); ESI-TOF-MS: [M+Na]+ m/z 491.0.
Methyl-6-(o-nitro-)benzamidyl-6-deoxy-2,3,4-tri-O-acetyl-α-D-mannopyranoside (3c). Yield: 91.3%; mp:162-164 °C; [a]D+28o (c 1.10, CHCl3); 1H-NMR δ (ppm) (CDCl3): 2.00 (s, 3H, Ac), 2.11 (s, 3H, Ac), 2.14 (s, 3H, Ac), 3.38 (s, 3H, OCH3), 3.60 (m, 1H, H-6e), 3.78 (m, 2H, H-6a, 5), 4.67 (s, 1H, H-1), 5.20-5.34 (m, 3H, H-2, 3, 4), 6.40 (t, 1H, J 6.6 Hz, H-NH), 7.58-8.09 (m, 4H, Ph); 13C-NMR δ (ppm) (CDCl3): 20.7(CH3CO), 20.8 (CH3CO), 39.8 (C-6), 55.3 (OCH3), 66.8 (C-4), 68.8 (C-5), 68.9 (C-3), 69.6 (C-2), 98.5 (C-1), 124.6 (Ph), 128.7 (Ph), 130.6 (Ph), 132.9 (Ph), 133.7 (Ph), 146.3(Ph), 166.4(C=O), 169.8 (CH3CO), 169.9 (CH3CO), 170.4 (CH3CO); ESI-TOF-MS: [M+1]+ m/z 469.0; [M+Na]+ m/z 491.0.
2-(o-Nitro)benzamidyl-2-deoxy-1,3,4,6-tetra-O-acetyl-β-D-glucopyranose (3d). Yield: 93.0%; mp: 144-148 °C; [a]D +40o (c 1.01, CHCl3); 1H-NMR δ (ppm) (CDCl3): 2.06 (s, 3H, Ac), 2.11 (s, 3H, Ac), 2.14 (s, 3H, Ac), 2.17 (s, 3H, Ac), 4.05-4.13 (m, 2H, H-6), 4.30 (dd, 1H, J 3.6 Hz, 12.6 Hz, H-4), 4.67 (m, 1H, H-5), 5.30 (m, 2H, H-2,3), 6.14 (d, 1H, J 8.4 Hz, H-1), 6.40 (d, 1H, J 3.6 Hz, H-NH), 7.27-8.09 (m, 4H, Ph);13C-NMR δ (ppm) (CDCl3): 20.5 (CH3CO), 20.7 (CH3CO), 20.8 (CH3CO), 20.9 (CH3CO), 51.9 (OCH3), 61.4 (C-sugar), 67.4 (C-sugar), 69.8 (C-sugar), 70.0 (C-sugar), 90.4(C-1), 124.6 (Ph), 128.7 (Ph), 130.8 (Ph), 132.0 (Ph), 134.0 (Ph), 145.9 (Ph), 166.5 (C=O), 168.7 (CH3CO), 169.9 (CH3CO), 170.7 (CH3CO), 172.4 (CH3CO); ESI-TOF-MS: [M+Na]+ m/z 519.1.
3.2.4. Synthesis and Spectral Data of 4a-c
The appropriate compound 3a-c (2.0 g, 7.4 mmol) was dissolved in THF (50 mL) and acetic acid (5 mL). Under stirring, zinc power (1.3 g, 20 mmol) was added slowly. The mixture was then refluxed for 2 h, cooled to room temperature, and filtered through a short column of silica gel. The eluent was evaporated to dryness under vacuum. The residue was dissolved in ethyl acetate and washed sequentially with saturated sodium hydrogen carbonate solution, saturated brine and water. The organic layer was dried over anhydrous Na2SO4 and evaporated under reduced pressure to afford the compounds 4a-c as yellow solids.
Methyl-6-(o-amino)benzamidyl-6-deoxy-2,3,4-tri-O-acetyl-α-D-glucopyranoside (4a). Yield: 88.0%; mp: 108-110 °C; [a]D +98o (c 1.01, CHCl3); 1H-NMR δ (ppm) (CDCl3): 2.01 (s, 3H, Ac), 2.07 (s, 3H, Ac), 2.09 (s, 3H, Ac), 3.43 (s, 3H, OCH3), 3.61 (m, 1H, H-6), 3.79 (m, 1H, H-5), 4.04 (m, 1H, H-6), 4.83 (dd, 1H, J1,2 3.6 Hz, J2,3 9.6 Hz, H-2), 4.93 (m, 2H, H-1, 3), 5.50 (t, 1H, J 9.6 Hz, H-3), 5.48 (t, 1H, J 9.9 Hz, H-4), 6.45 (t, 1H, H-NH), 6.45-7.36 (m, 4H, Ph); 13C-NMR δ (ppm) (CDCl3): 20.7 (CH3CO), 22.6 (CH3CO), 38.9 (C-6), 55.4 (OCH3), 67.7 (C-5), 69.7 (C-3), 69.9 (C-2), 70.9 (C-4), 96.6 (C-1), 112.2 (Ph), 114.1 (Ph), 127.4 (Ph), 132.9 (Ph), 148.8 (Ph), 169.7 (C=O), 170.0 (CH3CO), 170.1 (CH3CO), 170.2 (CH3CO); ESI-TOF-MS: [M+1]+ m/z 439.1; [M+Na]+ m/z 461.1.
Methyl-6-(o-amino)benzamidyl-6-deoxy-2,3,4-tri-O-acetyl-α-D-galactopyranoside (4b). Yield: 78.0%; mp: 78-80 °C; [a]D +28o (c 1.01, CHCl3); 1H-NMR δ (ppm) (CDCl3): 2.00 (s, 3H, Ac), 2.10 (s, 3H, Ac), 2.20 (s, 3H, Ac), 3.39 (s, 3H, OCH3), 3.44 (t, 1H, J 6.6 Hz, H-5), 3.60 (dd, 1H, J5,6e 6.9 Hz, J6a,6e 13.5 Hz, H-6e), 4.15 (dd, 1H, J5,6a 6.9 Hz, J6a, 6e 13.5 Hz, H-6a), 5.00 (d, 1H, J1, 2 3.6 Hz, H-1), 5.18 (dd, 1H, J1,2 3.6 Hz, J2,3 10.8 Hz, H-2), 5.37 (dd, 1H, J3,4 3.3 Hz, J2,3 10.8 Hz, H-3), 5.45 (d, 1H, J 3.3 Hz, H-4), 6.49 (t, 1H, J 6.3 Hz, H-NH), 6.63-7.33 (m, 4H, Ph); 13C-NMR δ (ppm) (CDCl3): 20.6 (CH3CO),20.7 (CH3CO), 20.8 (CH3CO), 38.8 (C-6), 55.0 (OCH3), 66.7 (C-5), 67.5 (C-3), 68.3 (C-2), 69.4 (C-4), 97.2 (C-1), 115.2 (Ph), 116.7 (Ph), 117.4 (Ph), 127.0 (Ph), 132.5 (Ph), 148.9 (Ph), 169.2 (C=O), 169.8 (CH3CO) , 170.5 (CH3CO), 171.0 (CH3CO); ESI-TOF-MS: [M+1]+ m/z 439.1; [M+Na]+ m/z 461.1.
Methyl-6-(o-amino)benzamidyl-6-deoxy-2,3,4-tri-O-acetyl-α-D-mannopyranoside (4c). Yield: 77.6%; mp: 150-154 °C; [a]D +40o (c 1.10, CHCl3); 1H-NMR δ (ppm) (CDCl3): 2.00 (s, 3H, Ac), 2.10 (s, 3H, Ac), 2.12 (s, 3H, Ac), 3.37 (s, 3H, OCH3), 3.40 (m, 1H, H-6e), 3.85-3.98 (m, 2H, H-5, 6a), 4.70 (s, 1H, H-1), 5.19-5.25 (m, 2H, H-2, 4), 5.36 (dd, 1H, J3,2 3.3 Hz, J3,4 10.8 Hz, H-3), 6.56 (t, 1H, J 5.4 Hz, H-NH), 6.63-7.37 (m, 4H, Ph); 13C-NMR δ (ppm) (CDCl3): 20.7 (CH3CO), 20.8 (CH3CO), 39.3 (C-6), 55.3 (OCH3), 67.1 (C-4), 68.8 (C-5), 68.9 (C-3), 69.6 (C-2), 98.4 (C-1), 115.8 (Ph), 116.5 (Ph), 117.3 (Ph), 126.9 (Ph), 132.4 (Ph), 148.8 (Ph), 169.2(C=O), 169.9 (CH3CO), 170.0 (CH3CO), 170.3 (CH3CO); ESI-TOF-MS: [M+1]+ m/z 439.1; [M+Na]+ m/z 461.1.
3.2.5. Synthesis and Spectral Data of 4d
Compound 3d (200 mg, 0.4 mmol) was dissolved in methanol (30 mL), and 40% Pd(OH)2 (20 mg) was added. Catalytic hydrogenation was carried out at 4.5 atm of pressure for 6 hours. The solid was filtered and the filtrate was evaporated to dryness to afford 180 mg of 4d, yield: 95.0%; mp: 158-160 °C; 1H-NMR δ (ppm) (CDCl3): 2.05 (s, 3H, Ac), 2.07 (s, 3H, Ac), 2.11 (s, 3H, Ac), 2.18 (s, 3H, Ac), 4.01-4.16 (m, 2H, H-5, 6), 4.30 (dd, 1H, J 3.6 Hz, 12.3Hz ), 4.67 (m, 1H, H-5), 5.26-5.42 (m, 2H), 6.25 (d, 1H, J 8.7 Hz, H-1), 6.31 (d, 1H, J 3.6 Hz, H-NH), 7.27-8.09 (m, 4H, Ph); 13C-NMR δ (ppm) (CDCl3) 20.6 (CH3CO), 20.7 (CH3CO), 20.8 (CH3CO), 20.9 (CH3CO), 51.3 (OCH3), 61.5, 67.4, 69.7, 70.6(C of sugar ring), 90.6 (C-1), 114.3 (Ph), 116.7 (Ph), 117.4 (Ph), 127.0 (Ph), 132.9 (Ph), 149.0 (Ph), 168.7 (C=O), 168.8 (CH3CO), 169.1 (CH3CO), 170.7 (CH3CO), 172.1 (CH3CO); ESI-TOF-MS: [M+1]+ m/z 467.1; [M+Na]+ m/z 489.1.
3.2.6. Synthesis and Spectral Data of 5a-d
Compounds 4a-d (300 mg) were dissolved in ClCH2CH2Cl (50 Ll), then triphosgene (140 mg, 0.54 mmol) was added. The mixture was refluxed for 6h and cooled to room temperature. CH2Cl2 (50 mL) was added and the organic layer was washed with saturated sodium hydrogen carbonate solution, saturated brine and water. The organic layer was dried over anhydrous Na2SO4, evaporated under reduced pressure to dryness, and purified with column chromatography on silica gel to yield white solids of 5a-d.
Methyl-6-(N3-)quinazolinedionyl-6-deoxy-2,3,4-tri-O-acetyl-α-D-glucopyranoside (5a). Yield: 89.0%; mp: 118-120 °C; [a]D +108o (c 1.01, CHCl3); 1H-NMR δ (ppm) (CDCl3): 2.01 (s, 3H, Ac), 2.05 (s, 3H, Ac), 2.18 (s, 3H, Ac), 3.20 (s, 3H, OCH3), 4.14 (dd, 1H, J6e, 5 3.9Hz, J6a, 6e 13.5 Hz, H-6e), 4.30 (m, 1H, H-5), 4.51 (dd, 1H, J6a, 5 8.4 Hz, J6a, 6e 12.9 Hz, H-6a), 4.90 (d, 1H, J1, 2 3.6 Hz, H-1), 4.94 (dd, 1H, J1,2 8.4 Hz, J2,312.9 Hz, H-2),5.09 (t, 1H, J 9.3 Hz, H-4), 5.48 (t, 1H, J 9.3 Hz, H-3), 7.16-8.16 (m, 4H, Ph). 10.2 (s, 1H, H-NH); 13C-NMR δ (ppm) (CDCl3): 20.7 (CH3CO), 39.4 (C-6), 55.0 (OCH3), 67.6 (C-5), 69.2 (C-3), 69.8 (C-2), 70.9 (C-4), 96.8 (C-1), 124.5 (Ph), 129.0 (Ph), 30.5 (Ph), 132.7 (Ph), 133.9 (Ph), 146.2 (Ph), 166.6 (C=O), 169.9 (CH3CO), 170.2 (CH3CO), 170.3 (CH3CO); ESI-TOF-MS: [M+1]+ m/z 465.0; [M+Na]+ m/z 487.0.
Methyl-6-(N3-)quinazolinedionyl-6-deoxy-2,3,4-tri-O-acetyl-α-D-galactopyranoside (5b). Yield: 89.0%, mp: 216-219 °C; [a]D +220o (c 1.01, CHCl3); 1H-NMR δ (ppm) (CDCl3): 1.96 (s, 3H, Ac), 2.08 (s, 3H, Ac), 2.26 (s, 3H, Ac), 3.34 (s, 3H, OCH3), 4.29 (m, 2H, H-5,6e), 4.52 (t, 1H, J 6.6 Hz, H-6a), 5.00 (d, 1H, J1, 2 3.3 Hz, H-1), 5.20 (dd, 1H, J1,2 3.3 Hz, J2,3 10.8 Hz, H-2), 5.33 (dd, 1H, J3,4 3.3 Hz, J2,3 10.8 Hz, H-3), 5.38 (d, 1H, J 3.0 Hz, H-4), 7.10-8.13 (m, 4H, Ph), 10.1 (s, 1H, H-NH); 13C-NMR δ (ppm) (CDCl3): 20.7 (s, 3H, Ac), 20.8 (s, 3H, Ac), 20.9 (CH3CO), 39.8 (C-6), 55.2 (OCH3), 65.2 (C-5), 67.9 (C-3), 68.1 (C-2), 68.2 (C-4), 97.0 (C-1), 114.2 (Ph), 115.0 (Ph), 123.6 (Ph), 128.5 (Ph), 135.3 (Ph), 138.4 (Ph), 151.6 (C=O), 162.2 (C=O), 170.1 (CH3CO), 170.4 (CH3CO), 170.8 (CH3CO); ESI-TOF-MS: [M+1]+ m/z 465.1; [M+Na]+ m/z 487.1.
Methyl-6-(N3-)quinazolinedionyl-6-deoxy-2,3,4-tri-O-acetyl-α-D-mannopyranoside (5c). Yield: 81.8%; mp: 78-82 °C; [a]D +32o (c 1.01, CHCl3); 1H-NMR δ (ppm) (CDCl3): 2.01 (s, 3H, Ac), 2.07, (s, 3H, Ac) 2.16 (s, 3H, Ac), 3.19 (s, 3H, OCH3), 4.14 (dd, 1H, J6e, 5 3.9 Hz, J6a, 6e 13.5 Hz, H-6e), 4.30 (m, 1H, H-5), 4.51 (dd, 1H, J6a, 5 8.4 Hz, J6a, 6e 13.5 Hz, H-6a), 4.60 (dd, 1H, J2,3 2.1 Hz, J1,2 4.8 Hz, H-2), 5.21 (d, 1H, J1, 2 2.1 Hz, H-1), 5.32-8.34 (m, 2H, H-4,3), 7.21-8.16 (m, 4H, Ph). 10.7 (s, 1H, H-NH); 13C-NMR δ (ppm) (CDCl3): 20.6 (CH3CO), 20.7 (CH3CO), 20.9 (CH3CO), 41.9 (C-6), 54.8 (OCH3), 67.3 (C-5), 69.0 (C-3), 69.1 (C-2), 69.5 (C-4), 98.1 (C-1), 114.1 (Ph), 115.2 (Ph), 123.5 (Ph), 128.4 (Ph), 135.2 (Ph), 138.5 (Ph), 152.0 (C=O), 162.2 (C=O), 169.9 (CH3CO), 170.1 (CH3CO), 170.2 (CH3CO); ESI-TOF-MS: [M+1]+ m/z 465.0; [M+Na]+ m/z 487.0.
2-(N3-)quinazolinedionyl-2-deoxy-1,3,4,6-tetra-O-acetyl-β-D-glucopyranoside (5d). Yield: 57.1%; mp: 193-195 °C; [a]D +88o (c 1.01, CHCl3); 1H-NMR δ (ppm) (CDCl3): 2.04 (s, 3H, Ac), 2.05 (s, 3H, Ac), 2.11 (s, 3H, Ac), 2.18 (s, 3H, Ac), 4.02-4.32 (m, 4H, H-sugar), 4.67 (m, 1H, H-5), 5.26-5.42 (m, 2H, H-sugar), 6.33 (d, 1H, J 3.6 Hz, H-sugar), 6.37 (d, 1H, J 8.1 Hz, H-1), 7.01-8.39 (m, 4H, Ph), 10.2 (ds, 1H, H-NH); 13C-NMR δ (ppm) (CDCl3): 20.6 (CH3CO), 20.7 (CH3CO), 20.8 (CH3CO), 20.9 (CH3CO), 51.8 (OCH3), 61.2, 61.5, 67.2, 69.7, 70.6 (C of sugar ring), 90.4 (C-1), 118.3 (Ph), 121.9 (Ph), 126.3 (Ph), 133.3 (Ph), 140.4 (Ph), 153.8 (Ph), 168.6 (C=O), 169.1 (CH3CO), 172.1 (CH3CO), 179.9 (C=O); ESI-TOF-MS: [M+NH4]+ m/z 510.0; [M+Na]+ m/z 515.0; [M+K]+ m/z 530.9.
3.2.7. Synthesis and Spectral Data of 6a-d
The appropriate intermediate 5a-d (130 mg) was dissolved in MeOH(20 mL) and sodium methoxide (10 mg, 0.18 mmol) was added and the mixture stirred for 30 min. The solution was then neutralized to pH 6-7 by with resin and filtered. The filtrate was evaporated to dryness to obtain light yellow solid of 6a-d.
Methyl-6-(N3-)quinazolinedionyl-6-deoxy-α-D-glucopyranoside (6a). Yield: 98%; mp: 137-142 °C; [a]D +60o (c 1.01, DMSO); 1H-NMR δ (ppm) (DMSO-d6): 2.97 (s, 3H, OCH3), 3.03 (m, 1H), 3.17 (m, 1H), 3.35 (m, 1H), 3.82 (m, 1H), 4.05 (m, 1H), 4.19 (m, 1H), 4.40 (d, 1H, J1, 2 3.0 Hz, H-1); 13C-NMR δ (ppm) (DMSO-d6): 41.8 (C-6), 53.7 (OCH3), 67.2 (C-5), 71.9 (C-3), 73.2 (C-2), 73.8 (C-4), 99.5 (C-1), 113.8 (Ph), 115.8 (Ph), 122.0 (Ph), 27.3 (Ph), 134.7 (Ph), 140.7(Ph), 150.9 (C=O), 162.3 (C=O); ESI-TOF-MS: [M+1]+ m/z 339.0; [M+Na]+ m/z 361.0.
Methyl-6-(N3-)quinazolinedionyl-6-deoxy-α-D-galactopyranoside (6b). Yield: 81.5%; mp: 235-237 °C; [a]D +220o (c 1.01, MeOH); 1H-NMR δ (ppm) (DMSO-d6): 3.34 (s, 3H, OCH3), 3.49-3.58 (m, 2H), 3.65-3.96 (m, 3H), 4.07 (dd, 1H, 1H, J2,3 3.6 Hz, J3,4 9.3 Hz, H-3), , 4.37 (s, 1H, H-1), 4.42-4.59 (m, 3H); 13C-NMR δ (ppm) (DMSO-d6): 40.9 (C-6), 55.2 (OCH3), 67.1, 68.2, 69.0, 70.2 (C of sugar ring), 99.9 (C-1), 104.2 (C-1), 113.7 (Ph), 115.1 (Ph), 122.4 (Ph), 127.4 (Ph), 135.0 (Ph), 139.4(Ph), 150.4 (C=O), 162.2 (C=O); ESI-TOF-MS: [M+1]+ m/z 339.1; [M+Na]+ m/z 361.0.
Methyl-6-(N3-)quinazolinedionyl-6-deoxy-α-D-mannopyranoside (6c). Yield: 81.0%; mp: 169-172oC; [a]D +60o (c 1.01, DMSO); 1H-NMR δ (ppm) (DMSO-d6): 2.93 (s, 3H, OCH3), 3.39-3.54 (m, 3H, H-5, 6a, 6e), 3.76 (m, 1H), 4.06 (dd, 1H, 1H, J2,3 3.6 Hz, J3,4 9.3 Hz, H-3), 4.27 (dd, 1H, J4,5 9.6 Hz, J3,4 13.2 Hz, H-4), 4.37 (s, 1H, H-1); 13C-NMR δ (ppm) (DMSO-d6): 41.8 (C-6), 53.4 (OCH3), 68.2, 70.1, 70.9 (C of sugar ring), 100.8 (C-1), 113.7 (Ph), 115.1 (Ph), 122.4 (Ph), 127.4 (Ph), 134.9 (Ph), 139.5 (Ph), 150.3 (C=O), 162.1 (C=O); ESI-TOF-MS: [M+1]+ m/z 339.0; [M+Na]+ m/z 361.0.
2-(N3-)quinazolinedionyl-2-deoxy-D-glucopyranoside (6d). Yield: 90.9%; mp:174-179 °C; [a]D +20o (c 1.10, DMSO); 1H-NMR δ (ppm) (DMSO-d6): 3.69-3.78 (m, 2H, H-5, H-6), 4.01 (dd, 1H, J6a, 5 3.0 Hz, J6a, 6e 5.4 Hz, H-6a), 4.60 (t, 1H, J 4.8 Hz), 4.78 (dd, 1H, J2,3 2.4 Hz, J2,1 5.1 Hz, H-2), 5.08 (dd, 1H, J2,3 2.4 Hz, J3,41 5.1 Hz, H-3), 5.54 (d, 1H, J2,1 5.1 Hz, H-1), 6.0 (s, 1H, H-OH), 7.18-7.95 (m, 4H, Ph); 13C-NMR δ (ppm) (DMSO-d6): 65.9, 69.3, 70.9, 80.6, 84.6 (C-sugar), 100.8 (C-1), 113.7 (Ph), 115.1 (Ph), 122.7 (Ph), 127.5 (Ph), 135.2 (Ph), 139.5 (Ph), 150.0 (C=O), 162.1 (C=O); ESI-TOF-MS: [M+NH4]+ m/z 338.0; [M+Na]+ m/z 347.0.