Experimental
General
Melting points were determined on Electrothermal Capillary melting point apparatus and are uncorrected. Thin-layer chromatography was performed with fluorescent silica gel plates HF254 Merck, which were checked under UV 254 and 365 nm light. The elemental analysis for C, H and N was done on a Perkin-Elmer Analyzer 2440. Infrared spectra (νmax-cm-1) were recorded on a Beckmann FT-IR 3303, using KBr disks. 1H-NMR spectra were recorded on JEOL EX-270 MHz NMR Spectrometer at 293 K in DMSO-d6. 13C-NMR spectra were recorded on a Varian Gemini at 50 MHz in DMSO-d6. Spectra were internally referenced to TMS. Peaks are reported in ppm downfield of TMS.
(7-Ethoxycarbonylmethoxy-2-oxo-2H-chromen-4-yl)-acetic acid methylester (2)
A mixture of (7-hydroxy-2-oxo-2H-chromen-4-yl)-acetic acid methylester (1, 25.74 g, 0.11 mole), anhydrous potassium carbonate (15.20 g, 0.11 mole) and ethyl bromoacetate (18.37 g, 0.11 mole) in dry acetone (200 mL) was refluxed with continuous stirring for 12 hours. After filtration, the solution was concentrated under reduced pressure, vacuum dried and the solid product was recrystallized from ethanol.M.p.185-186◦C, yield 64%; IR: νmax 3,429, 2,986, 2,941, 1,753, 1,724, 1,619, 1,439, 1,393, 1,341, 1,221, 1,198, 1,089 cm-1; 1H-NMR: δ 7.76 (d, 1H, H-5), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.92 (s, 2H, -OCH2), 4.19 (q, 2H, CH2, -CH2CH3), 4.02 (s, 2H, CH2), 3.65 (s, 3H, OCH3), 1.22 (t, 3H, CH3, -CH2CH3); 13C-NMR: δ 14.2 (CH2CH3), 34.8 (CH2CO), 52.1 (OCH3), 61.3 (CH2CH3), 65.5 (COCH2O), 109.6 (C-8), 112.8 (C-6), 113.8 (C-3), 114.8 (C-10), 128.3 (C-5), 151.2 (C-9), 155.2 (C-4), 160.3 (C-7), 160.9 (C-2), 168.9 (CO-O), 169.3 (C-CO-C); Anal. Calcd. for C16H16O7: C, 60.00; H, 5.04; Found: C, 59.98; H, 5.01%.
(7-Hydrazinocarbonylmethoxy-2-oxo-2H-chromen-4-yl)-acetic acid hydrazide (3)
To a solution of methanol (120 mL) and 86% hydrazine hydrate (12 mL) (7-ethoxycarbonylmethoxy-2-oxo-2H-chromen-4-yl)-acetic acid methylester (2, 3.2 g, 0.01 mole) was added, and the mixture was left to stand overnight at 5◦C. The product precipitated and was collected by suction filtration, washed with methanol (petrolether) and recrystallized from dil. acetic acid. M.p.> 300◦C, yield 70%; IR: νmax 3,461, 3,325 (NH), (NH2), 1,707 (lactone C=O), 1,623 (C=O, amide), 1,516 (C=C, arom.), 1,430, 1,298, 1,277 and 1,153 cm-1; 1H-NMR: δ 9.41 (s, 1H, NH), 9.34 (s, 1H, NH), 7.76 (d, 1H, H-5), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.94 (s, 2H, -OCH2), 4.34 (s, 2H, NH2), 4.08 (s, 2H, CH2), 3.38 (s, 2H, NH2); 13C-NMR: δ 45.8 (CH2), 68.9 (CH2O-), 108.0 (C-8), 111.8 (C-6), 112.9 (C-3), 114.1 (C-10), 128.3 (C-5), 152.2 (C-9), 155.2 (C-4), 160.4 (C-7), 160.9 (C-2), 166.8 (COCH2O), 169.6 (COCH2); Anal. Calcd. for C13H14N4O5: C, 50.98; H, 4.61; N, 18.29; Found: C, 51.02; H, 4.58; N, 18.25%.
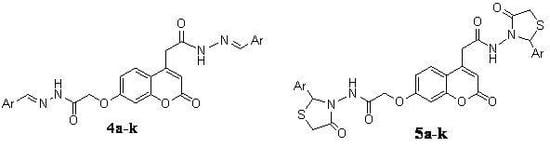
General procedure for preparation of (7-(arylidene-hydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl)-acetic acid aryilidene-hydrazides 4a-k
A mixture of (7-Hydrazinocarbonylmethoxy-2-oxo-2H-chromen-4-yl)-acetic acid hydrazide (3, 3.06 g, 0.01 mole) and appropriate aromatic aldehyde (Ar/a-k, 0.01 mole) was refluxed in absolute ethanol (30 mL) in the presence of a catalytic amount of glacial acetic for 2 to 4 hours. The reaction mixture was cooled, the solid separated was filtered and recrystallized from methanol to give compounds 4a-k.
[7-(Benzylidene-hydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl]-acetic acid benzylidene hydrazide (4a). M.p. 268-269◦C; yield 74%; IR: νmax 3,418, 3,313 (NH), 1,712, 1,682 (C=O, lactone), 1,666 (C=O, amide), 1,613 (C=C, arom., C=N, azomet.), 1,550, 1,378, 1,269 and 1,153 cm-1; 1H- NMR: δ 8.30 (s, 1H, HC=N-), 8.24 (s, 1H, HC=N-), 8.06 (s, 1H, NH), 8.02 (s, 1H, NH), 7.76 (d, 1H, H-5), 7.72-7.31 (m, 10H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.83 (s, 2H, -OCH2), 4.28 (s,2H,CH2); 13C-NMR: δ 45.7 (CH2), 69.2 (CH2O-), 108.1 (C-8), 111.6 (C-6), 112.5 (C-3), 114.4 (C-10), 127.9 (C-5), 128.4 (C-3,5, Ar-), 129.0 (C-2,6, Ar-), 131.4 (C-4, Ar-), 133.9 (C-1, Ar-), 143.6 (N=CH-), 151.8 (C-9), 155.2 (C-4), 160.4 (C-7), 160.9 (C-2), 166.9 (COCH2O), 170.0 (CONH-); Anal. Calcd. for C27H22N4O5: C, 67.21; H, 4.60; N, 11.61; Found: C, 67.19; H, 4.61; N, 11.58%.
[7-(2-Chlorobenzylidenehydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl]-acetic acid (2-chloro-benzylidene)- hydrazide (4b). M.p. 225-226◦C, yield (76%); IR: νmax 3,428, 3,283 (NH), 1,710, 1,692 (C=O, lactone), 1,656 (C=O, amide), 1,612 (C=C, arom., C=N, azomet.), 1,542, 1,398, 1,264 and 1,155 cm-1; 1H-NMR: δ 8.73 (s, 1H, -HC=N-), δ 8.62 (s, 1H, -HC=N-), δ 8.48 (s, 1H, NH), 8.45 (s, 1H, NH), 7.72 (d, 1H, H-5), 7.60-7.30 (m, 8H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.84 (s, 2H, -OCH2), 4.30 (s, 2H, CH2); 13C-NMR: δ 45.6 (CH2), 68.9 (CH2O-), 107.8 (C-8), 111.5 (C-6), 112.7 (C-3), 113.9 (C-10), 127.8 (C-5), 129.4 (C-3, Ar-), 130.6 (C-6, Ar-), 132.7 (C-4, Ar-), 133.8 (C-1, Ar-), 134.3 (C-2, Ar-), 143.5 (N=CH-), 151.7 (C-9), 155.0 (C-4), 160.3 (C-7), 160.9 (C-2), 166.4 (COCH2O), 169.9 (CONH-); Anal. Calcd. For C27H20Cl2N4O5: C, 58.81; H, 3.66; N, 10.16; Found: C, 58.79; H, 3.69; N, 10.12%.
[7-(3-Chlorobenzylidene-hydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl]-acetic acid (3-chloro-benzylidene)-hydrazide (4c). M.p. 259-261◦C, yield 72%; IR: νmax 3,408, 3,188 (NH), 1,727, 1,683 (C=O, lactone), 1,616 (C=O, amide, C=N, azomet.), 1,561 (C=C, arom.), 1,394, 1,262 and 1,138 cm-1; 1H-NMR: δ 8.31 (s, 1H, -HC=N-), 8.22 (s, 1H, -HC=N-), 8.48 (s, 1H, NH), 8.45 (s, 1H, NH), 7.76 (d, 1H, H-5), 7.67-7.30 (m, 8H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.84 (s, 2H, -OCH2), 4.28 (s, 2H, CH2); 13C-NMR: δ 45.5 (CH2), 69.1 (CH2O-), 107.9 (C-8), 111.4 (C-6), 112.5 (C-3), 113.4 (C-10), 127.3 (C-6, Ar-), 127.9 (C-5), 129.3 (C-2, Ar-), 130.3 (C-5, Ar-), 131.2 (C-4, Ar-), 135.2 (C-1, Ar-), 134.4 (C-3, Ar-), 143.4 (N=CH-), 151.6 (C-9), 155.2 (C-4), 160.6 (C-7), 160.9 (C-2), 166.7 (COCH2O), 169.8 (CONH-); Anal. Calcd. For C27H20Cl2N4O5: C, 58.81; H, 3.66; N, 10.16; Found: C, 58.80; H, 3.68; N, 10.13%.
[7-(2,4-Dihydroxy-benzylidene-hydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl]-acetic acid (2,4-dihydroxybenzylidene)-hydrazide (4d). M.p. 273-275◦C, yield 52%; IR: νmax 3,434 (OH), 3,366, 3,092 (NH), 1,712, 1,672 (C=O, lactone), 1,623 (C=O, amide, C=N, azomethine), 1,612 (C=C, arom., C=N), 1,559, 1,509, 1,395, 1,265 and 1,153 cm-1; 1H-NMR: δ 11.80 (s, 1H, OH), 11.17 (s, 1H, OH), 8.42 (s, 1H, -HC=N-), 8.30 (s, 1H, -HC=N-), 8.23 (s, 1H, NH), 8.19 (s, 1H, NH), 7.76 (d, 1H, H-5), 7.61-7.30 (m, 6H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.82 (s, 2H, -OCH2), 4.28 (s, 2H, CH2); 13C-NMR: δ 45.6 (CH2), 69.2 (CH2O-), 103.8 (C-3, Ar-), 107.6 (C-8), 108.7 (C-5, Ar-), 111.3 (C-6), 112.7 (C-3), 113.4 (C-10), 127.2 (C-6, Ar-), 127.9 (C-5), 135.2 (C-1, Ar-), 143.0 (N=CH-), 151.2 (C-9), 155.1 (C-4), 160.5 (C-7), 160.9 (C-2), 162.4 (C-2, Ar-), 162.6 (C-4, Ar-), 166.5 (COCH2O), 169.4 (CONH-); Anal. Calcd. For C27H22N4O9: C, 59.34; H, 4.06; N, 10.25; Found: C, 59.30; H, 4.07; N, 10.29%.
[7-(3,4-Dihydroxy-benzylidene-hydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl]- acetic acid (3,4-dihydroxybenzylidene)-hydrazide (4e). M.p. 205◦C, yield 62%; IR: νmax 3,408, 2,922 (NH), 1,725, 1,664 (C=O, lactone), 1,619 (C=O, amide, C=N, azomethine), 1,593 (C=C, arom.), 1,444, 1,393, 1,284 and 1,152 cm-1; 1H-NMR: δ 11.98 (s, 1H, OH), 11.45 (s, 1H, OH), 8.41 (s, 1H, -HC=N-), 8.30 (s, 1H, -HC=N-), 8.12 (s, 1H, NH), 8.03 (s, 1H, NH), 7.76 (d, 1H, H-5), 7.65-7.41 (m, 6H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.77 (s, 2H, -OCH2), 4.22 (s, 2H, CH2); 13C-NMR: δ 45.5 (CH2), 69.3 (CH2O-), 107.6 (C-8), 111.4 (C-6), 112.5 (C-3), 113.5 (C-10), 116.4 (C-2, Ar-), 117.5 (C-5, Ar-), 123.3 (C-6, Ar-), 127.8 (C-5), 127.9 (C-1, Ar-), 143.1 (N=CH-), 147.4 (C-3, Ar-), 149.6 (C-4, Ar-), 151.3 (C-9), 155.0 (C-4), 160.4 (C-7), 160.9 (C-2), 166.6 (COCH2O), 169.5 (CONH-); Anal. Calcd. For C27H22N4O9: C, 59.34; H, 4.06; N, 10.25; Found: C, 59.13; H, 4.03; N, 10.04%.
[7-(2,5-Dihydroxybenzylidene-hydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl]- acetic acid (2,5-dihydroxybenzylidene)-hydrazide (4f). M.p. 275-276◦C, yield 76%; IR: νmax 3,369, 3,286 (NH), 1,717, 1,681, 1,667 (C=O, lactone), 1,624 (C=O, amide, C=N, azomethine), 1,585 (C=C arom.), 1,492, 1,396, 1,267 and 1,156 cm-1; 1H-NMR: δ 11.95 (s, 1H,OH), 11.56 (s,1H,OH), 8.48 (s, 1H, -HC=N-), 8.34 (s, 1H, -HC=N-), 8.30 (s, 1H, NH), 8.25 (s, 1H, NH), 7.76 (d, 1H, H-5), 7.68-7.30 (m, 6H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.82 (s, 2H, -OCH2), 4.24 (s, 2H, CH2); 13C-NMR: δ 45.6 (CH2), 69.3 (CH2O-), 107.8 (C-8), 111.4 (C-6), 112.7 (C-3), 113.8 (C-10), 116.4 (C-6, Ar-), 117.4 (C-3, Ar-), 119.6 (C-4, Ar-), 119.9 (C-1, Ar-), 127.8 (C-5), 143.5 (N=CH-), 151.4 (C-9), 151.3 (C-5, Ar-), 153.7 (C-2, Ar-), 155.2 (C-4), 160.4 (C-7), 160.9 (C-2), 166.7 (COCH2O), 169.4 (CONH-); Anal. Calcd. For C27H22N4O9: C, 59.34; H, 4.06; N, 10.25; Found: C, 59.32; H, 4.04; N, 10.20%.
[7-(4-Hydroxy-3-methoxybenzylidene-hydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4yl]-acetic acid(4-hydroxy-3-methoxybenzylidene)-hydrazide (4g). M.p. 232-233◦C, yield 84%; IR: νmax 3,430, 3,224 (NH), 1,711, 1,671 (C=O, lactone), 1,622 (C=O, amide, C=N, azomethine), 1,605 (C=C, arom.), 1,429, 1,394, 1,272 and 1,164 cm-1; 1H-NMR: δ 11.96 (s, 1H, OH), δ 11.50 (s, 1H, OH), 8.19 (s, 1H, -HC=N-), 8.10 (s, 1H, -HC=N-), 7.99 (s, 1H, NH), 7.97 (s, 1H, NH), 7.77 (d, 1H, H-5), 7.40-7.21 (m, 6H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.78 (s, 2H, -OCH2), 4.24 (s, 2H, CH2), 3.80 (s, 6H, -OCH3); 13C-NMR: δ 45.6 (CH2), 56.0 (OCH3), 69.2 (CH2O-), 107.6 (C-8), 111.2 (C-6), 112.5 (C-3), 113.6( C-10), 114.8 (C-2, Ar-), 117.0 (C-5, Ar-), 122.9 (C-6, Ar-), 127.4 (C-1,Ar-), 127.8 (C-5), 143.3 (N=CH-), 148.1 (C-4, Ar-), 151.4 (C-9), 151.5 (C-3, Ar-), 155.1 (C-4), 160.4 (C-7), 160.9 (C-2), 166.8 (COCH2O), 169.8 (CONH-); Anal. Calcd. For C29H26N4O9: C, 60.62; H, 4.56; N, 9.75; Found: C, 60.59; H, 4.75; N, 9.70%.
[7-(3-Phenoxybenzylidene-hydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl]-acetic acid 3-phenoxybenzylidene)-hydrazide (4h). M.p. 236-237◦C, yield 57%; IR: νmax 3,409, 3,071 (NH), 1,726, 1,685 (C=O, lactone), 1,624 (C=O, lactone, C=N, azomethine), 1,597 (C=C, arom.), 1,490, 1,394, 1,261 and 1,156 cm-1; 1H-NMR: δ 8.30 (s, 1H, -HC=N-), 8.21 (s, 1H, -HC=N-), 8.03 (s, 1H, NH), 7.99 (s, 1H, NH), 7.76 (d, 1H, H-5), 7.70-7.10 (m, 18H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.79 (s, 2H, -OCH2), 4.18 (s, 2H, CH2); 13C-NMR: δ 45.5 (CH2), 56.1 (OCH3), 69.1 (CH2O-), 107.8 (C-8), 111.4 (C-6), 112.7 (C-3), 113.5 (C-10), 116.6 (C-2, Ar-), 117.5 (C-2,6, Ar-PhO), 119.8 (C-4, Ar-), 121.9 (C-4, Ar- PhO), 122.3 (C-2, Ar-), 127.8 (C-5), 128.5 (C-3,5 Ar- PhO), 128.9 (C-5 Ar-), 133.5 (C-1 Ar-), 143.4 (N=CH-), 151.3 (C-9), 155.4 (C-4), 157.1 (C-1 Ar- PhO), 157.1 (C-3 Ar-), 160.4 (C-7), 160.9 (C-2), 166.8 (COCH2O), 170.0 (CONH-); Anal. Calcd. For C39H30N4O7: C, 70.26; H, 4.54; N, 8.40; Found: C, 70.23; H, 4.55; N, 8.37%.
[7-(4-N,N-Dimethylaminobenzylidene-hydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl]-acetic acid (4-N,N-dimethylaminobenzylidene)-hydrazide (4i). M.p. 207-209◦C, yield 63%; IR: νmax 3,408, 3,082 (NH), 1,724, 1,679 (C=O, lactone), 1,623 (C=O, amide, C=N, azomethine), 1,604 (C=C, arom.), 1,554, 1,525, 1,364, 1,269 and 1,181cm-1; 1H-NMR: δ 8.49 (s, 1H, -HC=N-), 8.44 (s, 1H, -HC=N-), 8.17 (s, 1H, NH), 8.07 (s, 1H, NH), 7.66 (d, 1H, H-5), 7.24-7.52 (m, 8H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.74 (s, 2H, -OCH2), 4.18 (s, 2H, CH2), 3.32 (s, 6H, -N(CH3)2), 2.99 (s, 6H, -N(CH3)2); 13C-NMR: δ 40.3 (CH3N-), 45.6 (CH2), 69.1 (CH2O-), 107.6 (C-8), 111.3 (C-6), 112.7 (C-3), 113.8 (C-10), 114.4 (C-3,5 Ar-), 123.3 (C-1, Ar-), 127.8 (C-5), 130.2 (C-2,6 Ar-), 143.3 (N=CH-), 151.4 (C-9), 151.0 (C-4, Ar-), 155.5 (C-4), 160.6 (C-7), 160.9 (C-2), 166.7 (COCH2O), 169.8 (CONH-); Anal. Calcd. For C31H32N6O5: C, 64.97; H, 5.45; N, 15.15; Found: C, 65.89; H, 5.58; N, 15.11 %.
[7-(2-Hydroxy-5-nitrobenzylidenehydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl]-acetic acid (2-hydroxy-5-nitrobenzylidene)-hydrazide (4j). M.p. 204◦C, yield 82%; IR: νmax 3,367, 3,272 (NH), 1,706, 1,689 (C=O, lactone), 1,616 (C=O, C=N, azomethine), 1,600 (C=C, arom.), 1,577, 1,517, 1,481, 1,342, 1,287 and 1,150 cm-1; 1H-NMR: δ 12.02 (s, 2H, OH), 8.71 (s, 1H, -HC=N-), 8.59 (s, 1H, -HC=N-), 8.36 (s, 1H, NH), 8.31 (s, 1H, NH), 7.67 (d, 1H, H-5), 7.32-7.54 (m, 6H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.84 (s, 2H, -OCH2), 4.08 (s, 2H, CH2); 13C-NMR: δ 45.7 (CH2), 69.2 (CH2O-), 107.8 (C-8), 111.5 (C-6), 112.4 (C-3), 113.8 (C-10), 116.9 (C-3, Ar-), 119.4 (C-1, Ar-), 124.8 (C-4, Ar-), 125.5 (C-2, Ar-), 127.8 (C-5), 141.6 (C-5, Ar-), 143.4 (N=CH-), 151.4 (C-9), 155.4 (C-4), 160.8 (C-7), 160.9 (C-2), 166.2 (C-2, Ar-), 166.8 (COCH2O), 170.0 (CONH-); Anal. Calcd. For C27H20N6O11: C, 53.65; H, 3.33; N, 13.90; Found: C, 53.63; H, 3.35; N, 13.91%.
[2-Oxo-7-(3-phenylallylidenehydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl]-acetic acid (3-phenylallylidene)-hydrazide (4k). M.p. 290-292◦C, yield 68%; IR: νmax 3,428, 3,256 (NH), 1,718 (C=O, lactone), 1,624 (C=O, amide, C=N, azomethine), 1,613 (C=C, arom.), 1,560, 1,509, 1,393, 1,266 and 1,151 cm-1; 1H-NMR: δ 8.38 (s, 1H, -HC=N-), 8.24 (s, 1H, -HC=N-), 8.15 (s, 1H, NH), 8.08 (s, 1H, NH), 7.78 (2d, 4H, -HC=CH-), 7.64 (d, 1H, H-5), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 4.77 (s, 2H, -OCH2), 4.08 (s, 2H, CH2); 13C-NMR: δ 45.6 (CH2), 69.1 (CH2O-), 107.8 (C-8), 111.4 (C-6), 112.8 (C-3), 113.4 (C-10), 126.3 (C-2, Ar-), 126.4 (C-2,6, Ar-), 127.8 (C-5), 128.0 (C-4, Ar-), 128.9 (C-3,5, Ar-), 135.1 (C-1, Ar-), 139.0 (C-3, Ar-), 143.3 (N=CH-), 151.2 (C-9), 155.4 (C-4), 160.5 (C-7), 160.9 (C-2), 166.7 (COCH2O), 169.8,(CONH-); Anal. Calcd. For C31H26N4O5: C, 69.65; H, 4.90; N, 10.48; Found: C, 69.67; H, 4.88; N, 10.45%.
General procedure for the preparation of N-(2-aryl-4-oxo-thiazolidin-3-yl)-2-(4-(2-aryl-4-oxo-thiazolidin-3-ylcarbamoyl)-methyl)-2-oxo-2H-chromen-7-yloxy)-acetamides 5a-k
A mixture of (7-(arylidene-hydrazinocarbonylmethoxy)-2-oxo-2H-chromen-4-yl)-acetic acid aryilidenehydrazide 4a-k (0.01 mole) and mercaptoacetic acid (1.82 g, 0.02 mole) in DMF (30 mL) containing a pinch of anhydrous ZnCl2 was refluxed 6-8 hours. The reaction mixture was cooled and poured onto crushed ice. The solid thus obtained was filtered, washed with water and recrystallized from DMF yielding 5a-k.
2-{2-Oxo-7-[(4-oxo-2-phenylthiazolidin-3-ylcarbamoyl)-methoxy]-2H-chromen-4-yl}-N-(4-oxo-2-phenylthiazolidin-3-yl)acetamide (5a). M.p. 202-204◦C, yield 40%; IR: νmax 3,418, 3,313 (NH), 1,712, 1,682 (C=O, lactone), 1,666 (C=O, amide), 1,613 (C=C, arom.), 1,550, 1,378, 1,269 and 1,153 cm-1; 1H-NMR: δ 8.22 (s, 1H, -NH), 8.12 (s, 1H, -NH), 7.76 (d,1H,H-5), 7.71-7.23 (m, 10H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, -SCHN-), 4.83 (s, 2H, -OCH2), 4.28 (s, 2H, CH2), 3.38 (s, 2H, COCH2S-); 13C-NMR: δ 35.8 (COCH2S), 45.5 (CH2), 57.4 (NCHS), 69.1 (CH2O-), 107.6 (C-8), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 127.2 (C-4, Ar-), 127.8 (C-5), 128.7 (C-3,5, Ar-), 128.8 (C-2,6 Ar-), 139.2 (C-1, Ar-), 151.2 (C-9), 155.0 (C-4), 160.3 (C-7), 160.9 (C-2), 166.4 (COCH2O), 168.8 (SCH2CO-N), 173.3 (CONH-); Anal. Calcd. For C31H26N4O7S2: C, 59.94; H, 4.16; N, 8.88; S, 10.17; Found: C, 60.05; H, 4.14; N, 8.91; S, 10.14%.
N-[2-(2-Chlorophenyl)-4-oxo-thiazolidin-3-yl]-2-(7-{[2-(2-chlorophenyl)-4-oxo-thiazolidin-3-ylcarbamoyl]-methoxy}-2-oxo-2H-chromen-4-yl)-acetamide (5b). M.p. 184◦C, yield 76%; IR: νmax 3,425, 3,283 (NH), 1,692 (C=O, lactone), 1,656 (C=O, amide), 1,612 (C=C, arom.), 1,542, 1,398, 1,264 and 1,155 cm-1; 1H-NMR: δ 8.73 (s, 1H, NH-), 8.62 (s, 1H, NH-), 7.76 (d, 1H, H-5), 7.60-7.30 (m, 8H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, NCHS), 4.84 (s, 2H, -OCH2), 4.30 (s, 2H, CH2), 3.38 (s, 2H, COCH2S); 13C-NMR: δ 35.7 (COCH2S), 45.5 (CH2), 57.4 (NCHS), 69.10 (CH2O-), 107.6 (C-8), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 127.0 (C-5), 129.0 (C-3, Ar-), 130.6 (C-6, Ar-), 132.5 (C-4, Ar-), 133.4 (C-1, Ar-), 134.0 (C-2, Ar-), 143.0 (N=CH-), 151.2 (C-9), 155.0 (C-4), 160.3 (C-7), 160.9 (C-2), 166.4 (COCH2O), 173.0 (CONH-); Anal. Calcd. For C31H24Cl2N4O7S2: C, 53.22; H, 3.46; N, 8.01; S, 9.17; Found: C, 53.18; H, 3.44; N, 7.89; S, 9.20%.
N-[2-(3-Chlorophenyl)-4-oxo-thiazolidin-3-yl]-2-(7-{[2-(3-chlorophenyl)-4-oxo-thiazolidin-3-ylcarbamoyl]-methoxy}-2-oxo-2H-chromen-4-yl)-acetamide (5c). M.p. 240-241◦C, yield 72%; IR: νmax 3,450, 3,188 (NH), 1,727, 1,683 (CO, lactone), 1,616 (C=O, amide), 1,598 (C=C, arom.), 1,394, 1,262 and 1,138 cm-1; 1H-NMR: δ 8.31 (s, 1H, NH-), 8.22,(s, 1H, NH-), 7.76 (d, 1H, H-5), 7.67-7.30 (m, 8H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, NCHS), 4.84 (s, 2H, -OCH2), 4.28 (s, 2H, CH2), 3.38 (s, 2H, COCH2S); 13C-NMR: δ 35.7 (COCH2S), δ 45.5 (CH2), 57.4 (NCHS), 69.10 (CH2O-), 107.6 (C-8), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 127.3 (C-6, Ar-), 127.8 (C-5), 129.3 (C-2, Ar-), 130.3 (C-5, Ar-), 131.2 (C-4, Ar-), 135.2 (C-1, Ar-), 134.4 (C-3, Ar-), 143.0 (N=CH-), 151.2 (C-9), 155.0 (C-4), 160.3 (C-7), 160.9 (C-2), 173.0 (COCH2O), 173.0 (CONH-); Anal. Calcd. For C31H24Cl2N4O7S2: C, 53.22; H, 3.46; N, 8.01; S, 9.17; Found: C, 53.18; H, 3.44; N, 7.89; S, 9.20%.
N-[2-(2,4-Dihydroxyphenyl)-4-oxo-thiazolidin-3-yl]-2-(4-{[2-(2,4-dihydroxyphenyl)-4-oxo-thiazolidin-3-ylcarbamoyl]-methyl}-2-oxo-2H-chromen-7-yloxy)-acetamide (5d). M.p. 239-241◦C, yield 52%; IR: νmax 3,266 (OH), 3,092 (NH), 1,712, 1,672 (C=O, lactone), 1,624 (C=O, amide), 1,612 (C=C, arom.), 1,559, 1,509, 1,395, 1,265 and 1,153 cm-1; 1H-NMR: δ 11.80 (s, 1H, OH), 11.17 (s, 1H, OH), 8.42 (s, 1H, NH-), 8.30 (s, 1H, NH-), 7.76 (d, 1H, H-5), 7.61-7.30 (m, 6H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, NCHS), 4.82 (s, 2H, -OCH2), 4.28 (s, 2H, CH2), 3.38 (s, 2H, COCH2S); 13C-NMR: δ 35.7 (COCH2S), δ 45.5 (CH2), 47.4 (NCHS), 69.10 (CH2O-), 103.7 (C-3, Ar-), 107.6 (C-8), 108.4 (C-5, Ar-), 110.1 (C-1, Ar-), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 127.8 (C-5), 131.3 (C-6, Ar-), 151.2 (C-9), 157.2 (C-2, Ar-), 158.2 (C-4), 160.3 (C-7), 160.9 (C-2), 166.4 (CONH), 168.8 (NCOCH2), 173.3 (CH2CONH); Anal. Calcd. For C31H26N4O11S2: C, 53.60; H, 3.77; N, 8.07; S, 9.23; Found: C, 53.58; H, 3.79; N, 7.98; S, 9.20%.
N-[2-(3,4-Dihydroxyphenyl)-4-oxo-thiazolidin-3-yl]-2-(7-{[2-(3,4-dihydroxyphenyl)-4-oxo-thiazolidin-3-ylcarbamoyl]-methoxy}-2-oxo-2H-chromen-4-yl)-acetamide (5e). M.p. 198-200◦C, yield 47%; IR: νmax 3,388 (NH), 2,922 (OH), 1,725, 1,694 (C=O, lactone), 1,619 (C=O, amide), 1,523, 1,444, 1,393, 1,284 and 1,152 cm-1; 1H-NMR: δ 11.98 (s, 1H, OH), 11.45 (s, 1H, OH), 8.41 (s, 1H, NH), 8.30 (s, 1H, NH-), 7.76 (d, 1H, H-5), 7.65-7.41 (m, 6H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, NCHS), 4.77 (s, 2H, -OCH2), 4.22 (s, 2H, CH2), 3.38 (s, 2H, COCH2S); 13C-NMR: δ 35.7 (COCH2S), δ 45.5 (CH2), 57.4 (NCHS), 69.10 (CH2O-), 103.7 (C-3, from Ph), 107.6 (C-8), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 115.4 (C-2, Ar-), 117.4 (C-5, Ar-), 122.2 (C-6, Ar-), 117.8 (C-5), 133.8 (C-1, Ar-), 143.0 (N=CH-), 147.4 (C-3, Ar-), 145.6 (C-4, Ar-), 151.2 (C-9), 155.0 (C-4), 160.3 (C-7), 160.9 (C-2), 166.4 (CONH-), 168.8 (COCH2S), 173.3 (CH2CONH-); Anal. Calcd. For C31H26N4O11S2: C, 53.60; H, 3.77; N, 8.07; S, 9.23; Found: C, 53.58; H, 3.79; N, 7.98; S, 9.20%.
N-[2-(2,5-Dihydroxyphenyl)-4-oxo-thiazolidin-3-yl]-2-(7-{[2-(2,5-dihydroxyphenyl)-4-oxo-thiazolidin-3-ylcarbamoyl]-methoxy}-2-oxo-2H-chromen-4-yl)-acetamide (5f). M.p. 221-223◦C, yield 46%; IR: νmax 3,369 (OH), 3,286 (NH), 1,717, 1,681 (C=O, lactone), 1,667 (C=O, amide), 1,624 (C=C, arom.), 1,585, 1,492, 1,396, 1,267 and 1,156 cm-1; 1H-NMR: δ 11.95 (s, 1H, OH), 11.56 (s, 1H, OH), 8.48 (s, 1H, NH-), 8.34 (s, 1H, NH-), 7.76 (d, 1H, H-5), 7.68-7.30 (m, 6H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, NCHS), 4.82 (s, 2H, -OCH2), 4.24 (s, 2H, CH2), 3.38 (s, 2H, COCH2S); 13C-NMR: δ 35.7 (COCH2S), δ 45.5 (CH2), 47.4 (NCHS), 69.1 (CH2O-), 107.6 (C-8), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 115.4 (C-6, Ar-), 117.4 (C-3, Ar-), 115.6 (C-4, Ar-), 119.6 (C-1, Ar-), 127.8 (C-5), 143.0 (N=CH-), 148.7 (C-2, Ar-), 151.2 (C-9), 151.2 (C-5, Ar-), 155.0 (C-4), 160.2 (C-7), 160.9 (C-2), 166.4 (CONH-), 168.8 (COCH2S), 173.3 (CONH-); Anal. Calcd. For C31H26N4O11S2: C, 53.60; H, 3.77; N, 8.07; S, 9.23; Found: C, 53.58; H, 3.79; N, 7.98; S, 9.20%.
N-[2-(4-Hydroxy-3-methoxyphenyl)-4-oxo-thiazolidin-3-yl]-2-(7-{[2-(4-hydroxy-3-methoxyphenyl-4-oxo-thiazolidin-3-ylcarbamoyl]-methoxy}-2-oxo-2H-chromen-4-yl)-acetamide (5g). M.p. 217-218◦C, yield 84%; IR: νmax 3,434, 3,224 (NH), 1,711, 1,671 (C=O, lactone), 1,632 (C=O, amide), 1,603 (C=C, arom.), 1,529, 1,394, 1,272 and 1,164 cm-1; 1H-NMR: δ 11.96 (s, 1H, OH), 8.19 (s, 1H, NH-), 8.10 (s, 1H, NH-), 7.77 (d, 1H, H-5), 7.40-7.21 (m, 6H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, NCHS), 4.78 (s, 2H, -OCH2), 4.24 (s, 2H, CH2), 3.80 (s, 6H, -OCH3), 3.38 (s, 2H, COCH2S); 13C-NMR: δ 35.7 (COCH2S), δ 45.5 (CH2), 56.2 (OCH3), 57.8 (NCHS), 69.1 (CH2O-), 107.6 (C-8), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 114.8 (C-2, Ar-), 117.0 (C-5, Ar-), 122.9 (C-6, Ar-), 132.4 (C-1, Ar-), 144.1 (C-4, Ar-), 151.2 (C-9), 151.5 (C-3, Ar-), 155.0 (C-4), 160.3 (C-7), 160.9 (C-2), 166.4 (CONH-), 168.8 (COCH2S), 173.0 (CONH-), Anal. Calcd. For C33H30N4O11S2: C, 54.84; H, 4.18; N, 7.75; S, 8.87; Found: C, 54.79; H, 4.19; N, 7.71; S, 8.82%.
2-(2-Oxo-7-{[4-oxo-2-(3-phenoxyphenyl)-thiazolidin-3-ylcarbamoyl]-methoxy}-2H-chromen-4-yl)-N-[4-oxo-2-(3-phenoxyphenyl)-thiazolidin-3-yl]-acetamide (5h). M.p. 221-222◦C, yield 57%; IR: νmax 3,389, 3,071 (NH), 1,726, 1,685 (C=O, lactone), 1,628 (C=O, amide), 1,614 (C=C, arom.), 1,577, 1,490, 1,394, 1,261 and 1,156 cm-1; 1H-NMR: δ 8.30 (s, 1H, NH-), 8.21 (s, 1H, NH-), 7.76 (d, 1H, H-5), 7.70-7.10 (m, 18H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, NCHS), 4.79 (s, 2H, -OCH2), 4.18 (s, 2H, CH2), 3.38 (s, 2H, COCH2S); 13C-NMR: δ 35.7 (COCH2S), δ 45.5 (CH2), 56.2 (OCH3), 57.6 (NCHS), 69.10 (CH2O-), 107.6 (C-8), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 115.4 (C-4, Ar-), 116.1 (C-2, Ar-), 117.5 (C -2,6, Ar- PhO), 121.4 (C-4, Ar- PhO), 121.9 (C-6, Ar-), 127.8 (C-5), 128.5 (C-3,5 Ar- PhO), 128.6 (C-5 Ar-), 139.1 (C-1 Ar-), 151.2 (C-9), 155.0 (C-4), 156.8 (C-3, Ar-), 157.6 (C-1, Ar-PhO), 160.3 (C-7), 160.9 (C-2), 166.4 (CONH-), 168.9 (COCH2S), 173.3 (CH2CONH-); Anal. Calcd. For C43H34N4O9S2: C, 63.38; H, 4.21; N, 6.88; S, 7.87; Found: C, 63.34; H, 4.19; N, 6.86; S, 7.84%.
N-[2-(4-N,N-Dimethylaminophenyl)-4-oxo-thiazolidin-3-yl]-2-(7-{[2-(4-N,N-dimethylaminophenyl)-4-oxo-thiazolidin-3-ylcarbamoyl]-methoxy}-2-oxo-2H-chromen-4-yl)-acetamide (5i). M.p. 198-201◦C, yield 71%; IR: νmax 3,398, 3,082 (NH), 1,724, 1,679 (C=O, lactone), 1,623 (C=O, amide), 1,604 (C=C, arom.), 1,554, 1,525, 1,364, 1,269 and 1,181 cm-1; 1H-NMR: δ 8.49 (s, 1H, NH-), 8.44 (s, 1H, NH-), 7.66 (d, 1H, H-5), 7.24-7.52 (m, 8H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s,1H,H-3), 5.92 (s, 1H, NCHS), 4.74 (s, 2H, -OCH2), 4.18 (s, 2H, CH2), 3.38 (s, 2H, COCH2S), 3.32 (s, 6H, -N(CH3)2), 2.99 (s, 6H, -N(CH3)2); 13C-NMR: δ 35.7 (COCH2S), δ 40.3 (CH3N-), 45.5 (CH2), 57.6 (NCHS), 69.10 (CH2O-), 107.6 (C-8), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 114.4 (C-3,5, Ar-), 127.8 (C-5), 128.9 (C-1, Ar-), 130.1 (C-2,6 Ar-), 148.2 (C-4, Ar-), 151.2 (C-9), 155.0 (C-4), 160.3 (C-7), 160.9 (C-2), 166.4 (CONH-), 168.9 (COCH2S), 173.3 (CH2CONH-); Anal. Calcd. For C35H36N6O7S2: C, 58.64; H, 5.06; N, 11.72; S, 8.95; Found: C, 58.60; H, 4.98; N, 11.70; S, 8.90%.
N-[2-(2-Hydroxy-5-nitrophenyl)-4-oxo-thiazolidin-3-yl]-2-(4-{[2-(2-hydroxy-5-nitrophenyl)-4-oxo-thiazolidin-3-ylcarbamoyl]-methyl}-2-oxo-2H-chromen-7-yloxy)-acetamide (5j). M.p. 240-242◦C, yield 82%; IR: νmax 3,367, 3,272 (NH), 1,689 (C=O), 1,618 (C=O, amide), 1,598 (C=C, arom.), 1,577, 1,517, 1,481, 1,342, 1,287 and 1,150 cm-1; 1H-NMR: δ 12.02 (s, 2H, OH), 8.71 (s, 1H, NH-), 8.59 (s, 1H, NH-), 7.67 (d, 1H, H-5), 7.32-7.54 (m, 6H, arom.), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, NCHS), 4.84 (s, 2H, -OCH2), 4.08 (s, 2H, CH2), 3.38 (s, 2H, COCH2S); 13C- NMR: δ 35.7 (COCH2S), δ 45.5 (CH2), 47.6 (NCHS), 69.10 (CH2O-), 107.6 (C-8), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10), 116.9 (C-3, Ar-), 119.4 (C-1, Ar-), 121.8 (C-4, Ar-), 125.5 (C-6, Ar-), 127.8 (C-5), 141.1 (C-5, Ar-), 151.2 (C-9), 155.0 (C-4) 160.3 (C-7), 160.9 (C-2), 163.2 (C-2 Ar-), 166.4 (CONH-), 168.9 (COCH2S), 173.3 (CH2CONH-); Anal. Calcd. For C31H24N6O13S2: C, 49.47; H, 3.21; N, 11.17; S, 8.52; Found: C, 49.45; H, 3.19; N, 11.12; S, 8.50%.
2-{2-Oxo-7-[(4-oxo-2-styrylthiazolidin-3-ylcarbamoyl)-methoxy]-2H-chromen-4-yl}-N-(4-oxo-2-styrylthiazolidin-3-yl)-acetamide (5k). M.p. 221-224◦C, yield 48%; IR: νmax 3,424, 3,276 (NH), 1,718 (C=O, lactone), 1,628 (C=O, amide), 1,613 (C=C, arom.), 1,560, 1,509, 1,393, 1,266 and 1,151 cm-1; 1H-NMR: δ 8.38 (s, 1H, NH-), 8.24 (s, 1H, NH-), 7.78 (2d, 4H, -HC=CH-), 7.64 (d, 1H, H-5), 7.04 (d, 1H, H-6), 7.02 (s, 1H, H-8), 6.34 (s, 1H, H-3), 5.92 (s, 1H, NCHS), 4.77 (s, 2H, -OCH2), 4.08 (s, 2H, CH2), 3.38 (s, 2H, COCH2S); 13C-NMR: δ 36.3 (COCH2S), δ 45.5 (CH2), 56.9 (NCHS), 69.10 (CH2O-), 107.6 (C-8), 111.0 (C-6), 112.5 (C-3), 113.4 (C-10) 123.8 (C-1, ethenyl-Ar)), 126.4 (C-2,6, Ar-), 128.0 (C-4, Ar-), 128.7 (C-3,5, Ar-), 129.6 (C-2, ethenyl-Ar), 135.2 (C-1, Ar-), 137.3 (N=CH-), 139.0 (C-3 Ar-), 151.2 (C-9), 155.0 (C-4), 160.2 (C-7), 160.9 (C-2), 166.4 (CONH-), 168.9 (COCH2S), 173.3 (CH2CONH-); Anal. Calcd. For C35 H30 N4 O7S2: C, 61.57; H, 4.43; N, 8.21; S, 9.39;Found: C, 61.56; H, 4.41; N, 8.19; S, 9.40%.