Regioselective Suzuki-Miyaura Reaction: Application to the Microwave-promoted Synthesis of 4,7-Diarylquinazolines
Abstract
:Introduction
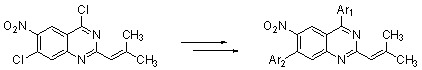
Results and Discussion


| Entry | Arylboronic acid Aryl- | Equiv. of Boronic acid | Yield % (6/7) |
|---|---|---|---|
| 1 | 4-MeO-Ph- | 4.0 | 0 / 70 |
| 2 | 4-MeO-Ph- | 1.5 | 45 / 15 |
| 3 | 4-MeO-Ph- | 1.2 | 68 / 0 |
| 4 | 4-Cl-Ph- | 4.0 | 0 / 67 |
| 5 | 4-Cl-Ph- | 2.0 | 42 / 13 |
| 6 | 4-Cl-Ph- | 1.5 | 63 / 0 |
| 7 | 3-CF3-Ph- | 1.5 | 48 / 0 |
| 8 | 5-CH3-2-thienyl- | 2.0 | 72 / 0 |
| 9 | 5-CH3-2-thienyl- | 1.5 | 61 / 0 |
| 10 | 3-NO2-Ph- | 2.0 | 55 / 0 |
| 11 | 3-NO2-Ph- | 1.5 | 43 / 0 |
| Entry | Aryl- | Product | Yield % |
|---|---|---|---|
| 1 | Ph- | 7f | 85 |
| 2 | 4-F-Ph- | 7g | 71 |
| 3 | 2-Tolyl- | 7h | 65 |

| Entry | Aryl 1 | Aryl 2 | Product | Yield % |
|---|---|---|---|---|
| 1 | 4-MeO-Ph- | 4-Cl-Ph- | 8 | 55 |
| 2 | 4-MeO-Ph- | Ph- | 9 | 64 |
| 3 | 4-MeO-Ph- | 4-F-Ph- | 10 | 43 |
| 4 | 4-MeO-Ph- | 2-Tolyl- | 11 | 50 |
| 5 | 4-MeO-Ph- | 3-CF3-Ph- | 12 | 57 |
| 6 | 4-Cl-Ph- | 4-MeO-Ph- | 13 | 54 |
| 7 | 4-Cl-Ph- | Ph- | 14 | 62 |
| 8 | 4-Cl-Ph- | 4-F-Ph- | 15 | 57 |
| 9 | 4-Cl-Ph- | 2-Tolyl- | 16 | 58 |
Experimental
General
Microwave instrumentation
Synthesis
7-Chloro-2-(chloromethyl)-6-nitroquinazolin-4(3H)-one (3)
7-Chloro-2-(2-methylprop-1-enyl)-6-nitroquinazolin-4(3H)-one (4)
4,7-Dichloro-2-(2-methylprop-1-enyl)-6-nitroquinazoline (5)
General procedure of the monocoupling and symmetric double coupling Suzuki-Miyaura reaction
General procedure of dissymmetric coupling Suzuki-Miyaura reaction of the 7-position
Conclusions
Acknowledgments
- Sample Availability: Samples of the compounds are available from the authors.
References and Notes
- Miyaura, N.; Yamada, K.; Suzuki, A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979, 20, 3437–3440. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Stereoselective synthesis of arylated (E)-alkenes by the reaction of alk-1-enylboranes with aryl halides in the presence of palladium catalyst. J. Chem. Soc. Chem. Commun. 1979, 866–867. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Miyaura, N. Organoboron Compounds. Top. Curr. Chem. 2002, 219, 11–59. [Google Scholar] [CrossRef]
- Suzuki, A.; Brown, H.C. Organic Syntheses via BoranesVolume 3: Suzuki coupling; Aldrich Chemical Company: Milwaukee, WI, USA, 2003. [Google Scholar]
- Suzuki, A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998. J. Organomet. Chem. 1999, 576, 147–168. [Google Scholar]
- Handy, S. T.; Sabatini, J.J. Regioselective Dicouplings: Application to Differentially Substituted Pyrroles. Org. Lett. 2006, 8, 1537–1537. [Google Scholar] [CrossRef]
- Tikad, A.; Routier, S.; Akssira, M.; Leger, J.-M.; Jarry, C.; Guillaumet, G. New Efficient Route to Dissymmetric 2,4-Di(het)aryl-pyrido[3,2-d]pyrimidines via Regioselective Cross-Coupling Reactions. Org. Lett. 2007, 9, 4673–4676. [Google Scholar] [CrossRef]
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 1999, 16, 697–709. [Google Scholar] [CrossRef]
- Sirisoma, N.; Kasibhatla, S.; Pervin, A.; Zhang, H.; Jiang, S.; Willardsen, J.A.; Anderson, M.B.; Baichwal, V.; Mather, G.G.; Jessing, K.; Hussain, R.; Hoang, K.; Pleiman, C.M.; Tseng, B.; Drewe, J.; Cai, S.X. Discovery of 2-Chloro-N-(4-methoxyphenyl)-N-methylquinazolin-4-amine (EP128265, MPI-0441138) as a Potent Inducer of Apoptosis with High In Vivo Activity. J. Med. Chem. 2008, 51, 4771–4779. [Google Scholar]
- Foote, K.M.; Mortlock, A.A.; Heron, N.M.; Jung, F.H.; Hill, G.B.; Pasquet, G.; Brady, M.C.; Green, S.; Heaton, S.P.; Kearney, S.; Keen, N.J.; Odedra, R.; Wedgea, S.R.; Wilkinsona, R.W. Synthesis and SAR of 1-acetanilide-4-aminopyrazole-substituted quinazolines: Selective inhibitors of Aurora B kinase with potent anti-tumor activity. Bioorg. Med. Chem. Lett. 2008, 18, 1904–1909. [Google Scholar]
- Barlaam, B.; Acton, D.G.; Ballard, P.; Bradbury, R.H.; Cross, D.; Ducray, R.; Germain, H.; Hudson, K.; Klinowska, T.; Magnien, F.; Ogilvie, D.J.; Olivier, A.; Ross, H.S.; Smith, R.; Trigwell, C.B.; Vautier, M.; Wright, L. Neutral 5-substituted 4-indazolylaminoquinazolines as potent, orally active inhibitors of erbB2 receptor tyrosine kinase. Bioorg. Med. Chem. Lett. 2008, 18, 1799–1803. [Google Scholar]
- Chandrika, P.M.; Yakaiah, T.; Ram Rao, A.R.; Narsaiah, B.; Reddy, N.C.; Sridhar, V.; Rao, J.V. Synthesis of novel 4,6-disubstituted quinazoline derivatives, their anti-inflammatory and anti-cancer activity (cytotoxic) against U937 leukemia cell lines. Eur. J. Med. Chem. 2008, 43, 846–852. [Google Scholar]
- Verhaeghe, P.; Azas, N.; Gasquet, M.; Hutter, S.; Ducros, C.; Laget, M.; Rault, S.; Rathelot, P.; Vanelle, P. Synthesis and antiplasmodial activity of new 4-aryl-2-trichloromethylquinazolines. Bioorg. Med. Chem. Lett. 2008, 18, 396–401. [Google Scholar]
- Verhaeghe, P.; Azas, N.; Hutter, S.; Castera-Ducros, C.; Laget, M.; Dumètre, A.; Gasquet, M.; Reboul, J.-P.; Rault, S.; Rathelot, P.; Vanelle, P. Synthesis and in vitro antiplasmodial evaluation of 4-anilino-2-trichloromethylquinazolines. Bioorg. Med. Chem. 2009, 17, 4313–4322. [Google Scholar] [CrossRef]
- Kabri, Y.; Azas, N.; Dumètre, A.; Hutter, S.; Laget, M.; Verhaeghe, P.; Gellis, A.; Vanelle, P. Original quinazoline derivatives displaying antiplasmodial properties. Eur. J. Med. Chem. 2010, 45, 616–622. [Google Scholar]
- Dallinger, D.; Kappe, C.O. Microwave-Assisted Synthesis in Water as Solvent. Chem. Rev. 2007, 107, 2563–2591. [Google Scholar] [CrossRef]
- Gong, Y.; He, W. Direct Synthesis of Unprotected 4-Aryl Phenylalanines via the Suzuki Reaction under Microwave Irradiation. Org. Lett. 2002, 4, 3803–3805. [Google Scholar] [CrossRef]
- Arvela, R.K.; Leadbeater, N.E. Microwave-Promoted Heck Coupling Using Ultralow Metal Catalyst Concentrations. J. Org. Chem. 2005, 70, 1786–1790. [Google Scholar] [CrossRef]
- Gellis, A.; Boufatah, N.; Vanelle, P. Rapid microwave-promoted synthesis of new sulfonylmethylbenzothiazoles in water. Green Chem. 2006, 8, 483–487. [Google Scholar] [CrossRef]
- Villemin, D.; Caillot, F. Microwave mediated palladium-catalysed reactions on potassium fluoride/alumina without use of solvent. Tetrahedron Lett. 2001, 42, 639–642. [Google Scholar] [CrossRef]
- Varma, R.S. Solvent-free accelerated organic syntheses using microwaves. Pure Appl. Chem. 2001, 73, 193–198. [Google Scholar] [CrossRef]
- Cvengros, A.; Toma, S.; Marque, S.; Loupy, A. Synthesis of phosphonium salts under microwave activation — Leaving group and phosphine substituents effects. Can. J. Chem. 2004, 82, 1365–1371. [Google Scholar] [CrossRef]
- He, P.; Haswell, S.J.; Fletcher, D.I. Microwave-assisted Suzuki reactions in a continuous flow capillary reactor. Appl. Catal. A Gen. 2004, 274, 111–114. [Google Scholar] [CrossRef]
- Genov, M.; Almorin, A.; Espinet, P. Microwave assisted asymmetric Suzuki-Miyaura and Negishi cross-coupling reactions: Synthesis of chiral binaphthalenes. Tetrahedron Asymmetry 2007, 18, 625–627. [Google Scholar] [CrossRef]
- Kabri, Y.; Gellis, A.; Vanelle, P. Synthesis of original 2-substituted 4-arylquinazolines by microwave-irradiated Suzuki-Miyaura cross-coupling reactions. Eur. J. Org. Chem. 2009, 4059–4066. [Google Scholar]
- Mangalagiu, I.; Benneche, T.; Undheim, K. Trialkylalanes in palladium-catalyzed chemo- and regioselective alkylations. Tetrahedron Lett. 1996, 37, 1309–1312. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; McMillen, W.T.; Chatterjee, A.; Toth, J.E.; Mundla, S.R.; Voss, M.; Boyer, R.D.; Sawyer, J.S. A concise synthesis of quinazolinone TGF-β RI inhibitor through one-pot three-component Suzuki–Miyaura/etherification and imidate–amide rearrangement reactions. Tetrahedron 2007, 63, 11763–11770. [Google Scholar]
- Littke, A.F.; Fu, G.C. Palladium-Catalyzed Coupling Reactions of Aryl Chlorides. Angew. Chem. Int. Ed. 2002, 41, 4176–4211. [Google Scholar] [CrossRef]
- Kabri, Y.; Gellis, A.; Vanelle, P. Microwave-assisted synthesis in aqueous medium of new quinazoline derivatives as anticancer agent precursors. Green Chem. 2009, 11, 201–208. [Google Scholar] [CrossRef]
- Tworowski, D.; Matsievitch, R. Preparation of piperazine derivatives for treatment of sexual disorders. PCT Int. Appl. WO 2007110868, 2007. [Chem. Abstr. 2007, 147, 427370]. [Google Scholar]
- Crozet, M.P.; Gellis, A.; Pasquier, C.; Vanelle, P.; Aune, J.-P. Electron transfer reactivity in 5-nitrouracil series. Tetrahedron Lett. 1995, 36, 525–528. [Google Scholar]
- Gellis, A.; Vanelle, P.; Kaafarani, M.; Benakli, K.; Crozet, M.P. Synthèse et réactions SRN1 en série 5-nitrothiazole. Tetrahedron 1997, 53, 5471–5484. [Google Scholar]
- Conolly, D.J.; Lacey, P.M.; Mc Carthy, M.; Saunders, C.P.; Carroll, A.-M.; Goddard, R.; Guiry, P.J. Preparation and Resolution of a Modular Class of Axially Chiral Quinazoline-Containing Ligands and Their Application in Asymmetric Rhodium-Catalyzed Olefin Hydroboration. J. Org. Chem. 2004, 69, 6572–6589. [Google Scholar] [CrossRef]
- Pedzisa, L.; Vaughn, I.W.; Pongdee, R. Suzuki–Miyaura cross-coupling of α-phosphoryloxy enol ethers with arylboronic acids. Tetrahedron Lett. 2008, 49, 4142–4144. [Google Scholar] [CrossRef]
- Eichenberger, T.; Ruch, T. Bulk dyeing of polymeric material with polycyclic compounds, and the dyes used. Eur. Pat. Appl. EP 763538, 1997. [Google Scholar]
© 2010 by the authors;
Share and Cite
Kabri, Y.; Verhaeghe, P.; Gellis, A.; Vanelle, P. Regioselective Suzuki-Miyaura Reaction: Application to the Microwave-promoted Synthesis of 4,7-Diarylquinazolines. Molecules 2010, 15, 2949-2961. https://doi.org/10.3390/molecules15052949
Kabri Y, Verhaeghe P, Gellis A, Vanelle P. Regioselective Suzuki-Miyaura Reaction: Application to the Microwave-promoted Synthesis of 4,7-Diarylquinazolines. Molecules. 2010; 15(5):2949-2961. https://doi.org/10.3390/molecules15052949
Chicago/Turabian StyleKabri, Youssef, Pierre Verhaeghe, Armand Gellis, and Patrice Vanelle. 2010. "Regioselective Suzuki-Miyaura Reaction: Application to the Microwave-promoted Synthesis of 4,7-Diarylquinazolines" Molecules 15, no. 5: 2949-2961. https://doi.org/10.3390/molecules15052949
APA StyleKabri, Y., Verhaeghe, P., Gellis, A., & Vanelle, P. (2010). Regioselective Suzuki-Miyaura Reaction: Application to the Microwave-promoted Synthesis of 4,7-Diarylquinazolines. Molecules, 15(5), 2949-2961. https://doi.org/10.3390/molecules15052949