Chitosan Based Heterogeneous Catalyses: Chitosan-Grafted-Poly(4-Vinylpyridne) as an Efficient Catalyst for Michael Additions and Alkylpyridazinyl Carbonitrile Oxidation
Abstract
:1. Introduction


2. Results and Discussion
2.1. Preparation of Cs-PVP Catalyst Beads


2.2. Applying Cs-PVP Beads as Efficient Basic Heterogeneous Catalyst in Michael Additions




| Product | Fresh Catalyst | Recycled (1) | Recycled (2) | Recycled (3) | Recycled (4) |
|---|---|---|---|---|---|
| 2 | 95 | 93 | 93 | 92 | 92 |

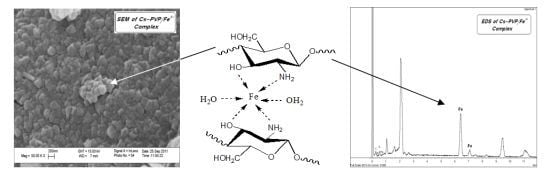
2.3. Preparation of Cs-PVP/Fe(III) Catalyst Beads
| Element | Weight% | Atomic% |
|---|---|---|
| C K | 17.24 | 38.89 |
| N K | 4.25 | 8.22 |
| O K | 12.25 | 20.74 |
| Fe K | 66.27 | 32.15 |
| Totals | 100.00 |


2.4. Utility of Cs-PVP/Fe(III) beads as an Efficient Catalyst for Oxidation of Methyl Pyridazinones

3. Experimental
3.1. General
3.2. Samples
3.3. Materials
3.3.1. Preparation of Chitosan Beads Cs
3.3.2. Preparation of Basic Catalyst Chitosan-g-poly(vinylpyridine)(Cs-PVP) Beads
3.3.2.1. Heterogeneous Grafting of 4-Vinylpyridine onto Chitosan
3.3.2.2. Preparation of Chitosan/PVP Copolymer Beads
3.3.3. Preparation of Cs-PVP—Supported Iron(III) Complex by Adsorption of Fe3+ Ions on Cs-PVP Grafted Chitosan Beads Using Fe(NO3)3 [34]

3.3.4. Reaction of Benzylidene-Malononitrile 1 with Dimedone and Cyclohexanone
3.3.5. Reaction of Arylidene-Malononitrile with Ethyl Acetoacetate
3.3.6. Reaction of 1 with α-Naphthol
3.3.7. One Pot Reaction of benzaldehyde and malononitrile with 2-cyanothioacetamide (11)
3.3.8. Reaction of 1 with 4,4-Dimethoxybutan-2-one
3.3.9. Reaction of Enaminones 14a,b with Malononitrile
3.3.10. Oxidation of Methyl Pyridazinone with Cs-PVP-Fe(III) Complex
4. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- Handbook of Green Chemistry and Technology; Clark, J.; Macquarrie, D. (Eds.) Blackwell Science Ltd.: Oxford, UK, 2002.
- Gavrilescu, M.; Chisti, Y. Biotechnology—A sustainable alternative for chemical industry. Biotechnol. Adv. 2005, 23, 471–499. [Google Scholar] [CrossRef]
- Brundtland, G.H. Our Common Future, World Commission on Environment and Development; Oxford University Press: Oxford, UK, 1987. [Google Scholar]
- Kunbeck, D.; Saidulu, G.; Reddy, K.R.; Diaz, D. Critical assessment of the efficiency of chitosan biohydrogel beads as recyclable and heterogeneous organocatalyst for C–C bond formation. Green Chem. 2012, 14, 378–392. [Google Scholar] [CrossRef] [Green Version]
- Bommarius, A.S.; Riebel, B.R. Biocatalysis: Fundamentals and Applications; Wiley-VCH: Weinheim, Germany, 2004; p. 624. [Google Scholar]
- Nemtsev, S.V.; Gamzazade, A.I.; Rogozhin, S.V.; Bykova, V.M.; Bykov, V.P. Deacetylation of chitin under homogeneous conditions. Applied Biochem. Microbiol. 2002, 38, 521–526. [Google Scholar] [CrossRef]
- Udomchai, C.; Piyabutr, W.; Chuen, H.N.; Willem, S.F.; Suwalee, C. Chemical Deacetylation of Shrimp Chitin in different Conditions. Adv. Chitin Sci. 1998, 3, 165–168. [Google Scholar]
- Tajik, H.; Moradi, M.; Rohani, S.M.R.; Erfani, A.M.; Jalali, F.S.S. Preparation of Chitosan from Brine Shrimp (Artemia urmiana) Cyst Shells and Effects of Different Chemical Processing Sequences on the Physicochemical and Functional Properties of the Product. Molecules 2008, 13, 1263–1274. [Google Scholar] [CrossRef]
- Al-Matar, H.M.; Khalil, K.D.; Meier, H.; Kolshorn, H.; Elnagdi, M.H. Chitosan as heterogeneous catalyst in Michael additions: the reaction of cinnamonitriles with active methylene moieties and phenols. ARKIVOC 2008, 16, 288–301. [Google Scholar]
- Gomha, S.M.; Riyadh, S.M. Synthesis of triazolo[4,3-b][1,2,4,5]tetrazines and triazolo[3,4-b][1,3,4]thiadiazines using chitosan as ecofriendly catalyst under microwave irradiation. ARKIVOC 2009, 11, 58–68. [Google Scholar]
- Khalil, K.D.; Al-Matar, H.M.; Elnagdi, M.H. Chitosan as an eco-friendly heterogeneous catalyst for Michael type addition reactions. A simple and efficient route to pyridones and phthalazines. Eur. J. Chem. 2010, 1, 252–258. [Google Scholar] [CrossRef]
- Hardy, J.E.; Hubert, S.; Macquarrie, D.J.; Wilson, A.J. Chitosan-based heterogeneous catalysts for Suzuki and Heck reactions. Green Chem. 2004, 6, 53–56. [Google Scholar] [CrossRef]
- Elnagdi, M.H.; Abed, N.M.; Elmoghayar, M.R.H.; Fleta, D.H. Reactivity of α-cyanochelkones as Michael acceptor. Indian J. Chem. 1976, 14B (6), 422–424. [Google Scholar]
- Abdou, S.; Fahmy, S.M.; Sadek, K.U.; Elnagdi, M.H. Activated nitriles in heterocyclic synthesis: A novel synthesis of pyrano[2,3-c]pyrazoles. Heterocycles 1981, 16, 2177–2180. [Google Scholar] [CrossRef]
- Elnagdi, M.H.; Maksoud, F.A.; Abdel-Aal, F.A.; Hafez, E.A.; Yassin, Y.M. Studies on alkyl heterocyclic aromatic compounds. New route for synthesis of Polyazana-phthalenes. Z. Naturforsch. 1989, 44B (6), 683–689. [Google Scholar]
- Elagamey, A.A.; El-Taweel, F.M.A.; Sowellim, S.Z.; Sofan, M.A.; Elnagdi, M.H. Nitriles in heterocyclic synthesis: A novel route for the synthesis of naphthalopyrans, pyridines 2H-and 4H-pyrans. Collect. Czech. Chem. Commun. 1990, 55, 524–534. [Google Scholar] [CrossRef]
- Elnagdi, M.H.; Ghozlan, S.A.S.; Abdelhamid, I.A. Alkyl-substituted heteroaromatics as precursors to polycyclic heteroaromatics: recent developments. ARKIVOC 2008, 10, 54–84. [Google Scholar]
- Fu, C.; Hung, T.; Su, C.; Suryani, D.; Wu, W.; Dai, W.; Yeh, Y. Immobilization of calcium oxide onto chitosan beads as a heterogeneous catalyst for biodiesel production. Polym. Int. 2011, 60, 957–962. [Google Scholar] [CrossRef]
- Shamim, T.; Paul, S. Silica Functionalized Cu(I) as a Green and Recyclable Heterogeneous Catalyst for the Huisgen 1,3-Dipolar Cycloaddition in Water at Room Temperature. Catal. Lett. 2010, 136, 260–265. [Google Scholar] [CrossRef]
- Ricci, A.; Bernardi, L.; Gioia, C.; Vierucci, S.; Robitzer, M.; Quignard, F. Chitosan aerogel: a recyclable, heterogeneous organocatalyst for the asymmetric direct aldol reaction in water. Chem. Commun. 2010, 46, 6288–6290. [Google Scholar] [CrossRef]
- Qin, Y.; Zhao, W.; Yang, L.; Zhang, X.; Cui, Y. Chitosan-based heterogeneous catalysts for enantioselective Michael reaction. Chirality 2012, 24, 640–645. [Google Scholar] [CrossRef]
- Dekamin, M.G.; Azimoshan, M.; Ramezani, L. Chitosan: A Highly Efficient Renewable and Recoverable Bio-Polymer Catalyst for Expeditious Synthesis of α-Amino nitriles and Imines under Mild Conditions. Green Chem. 2013, in press. [Google Scholar]
- Watile, R.A.; Bhanage, B.M. Chitosan biohydrogel beads: A recyclable, biodegradable, heterogeneous catalyst for the regioselective synthesis of 5-aryl-2-oxazolidinones from carbon dioxides and aziridines at mild conditions. Ind. J. Chem. 2012, 51A, 1354–1360. [Google Scholar]
- Yuanchen, C.; Hefeng, Z.; Runtao, L.; Yi, L.; Chu, X. Asymmetric Henry reaction catalyzed by chitosan and its L-proline derivatives. Chin. J. Org. Chem. 2010, 30, 707–712. [Google Scholar]
- Chtchigrovsky, M.; Primo, A.; Gonzalez, P.; Molvinger, K.; Robitzer, M.; Quignard, F.; Taran, F. Functionalized chitosan as a green, recyclable, biopolymer-supported catalyst for the (3+2) Huisgen cycloaddition. Angrew. Chem. 2009, 48, 5916–5920. [Google Scholar] [CrossRef]
- Elkholy, S.S.; Khalil, K.D.; Elsabee, M.Z. Homogeneous and heterogeneous grafting of 4-vinylpyridine onto chitosan. J. Appl. Poly. Sci. 2006, 99, 3308–3317. [Google Scholar] [CrossRef]
- Barriiro-Iglesias, R.; Coronilla, R.; Concheiro, A.; Alvarez-Lorenzo, C. Preparation of chitosan beads by simultaneous cross-linking/insolubilisation in basic pH. Rheological optimisation and drug loading/release behavior. Euro. J. Pharm. Sci. 2005, 24, 77–84. [Google Scholar]
- Guibal, E. Heterogeneous catalysis on chitosan-based materials: a review. Prog. Polym. Sci. 2005, 30, 71–109. [Google Scholar] [CrossRef]
- Guibal, E.; Milot, C.; Tobin, J.M. Palladium sorption using chitosan derivatives. Ind. Eng. Chem. Res. 1998, 37, 1454–1463. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Endud, C.S.; Mayanar, R. Removal of copper(II) ions from aqueous solution onto chitosan and crosslinked chitosan beads. React. Funct. Polym. 2002, 50, 181–190. [Google Scholar] [CrossRef]
- Yang, T.C.; Zall, R.R. Adsorption of metals by natural polymers generated from sea food processing wastes. Ind. Eng. Chem. Prod. Res. Dev. 1984, 23, 168. [Google Scholar] [CrossRef]
- Varma, A.J.; Deshpande, S.V.; Kennedy, J.F. Metal complexation by chitosan and its derivatives: a review. Carbohydr. Polym. 2004, 55, 77–93. [Google Scholar] [CrossRef]
- Wan Ngah, W.S.; Ghani, S.Ab.; Kamari, A. Adsorption behaviour of Fe(II) and Fe(III) ions in aqueous solution on chitosan and cross-linked chitosan beads. Bioresour. Technol. 2004, 96, 443–450. [Google Scholar]
- Burke, A.; Yilmaz, E.; Hasirci, N. Evaluation of Chitosan as a Potential Medical Iron (III) Ion Adsorbent. Turk. J. Med. Sci. 2000, 30, 341–348. [Google Scholar]
- Crystallographic data for the structures of compounds 2, and 4 reported in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publications Nos. 895828 and 895829. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre. Available online: http://www.ccdc.cam.ac.uk (accessed on 6 May 2013).
- Elnagdi, N.M.H.; Al-Hokbany, N.S. Organocatalysis in Synthesis: L-Proline as an Enantioselective Catalyst in the Synthesis of Pyrans and Thiopyrans. Molecules 2012, 17, 4300–4312. [Google Scholar] [CrossRef]
- Alnajjar, A.; Abdelkhalik, M.M.; Al-Enezi, A.; Elnagdi, M.H. Enaminones as Building Blocks in Heterocyclic Syntheses: Reinvestigating the Product Structures of Enaminones with Malononitrile. A Novel Route to 6-Substituted-3-Oxo-2,3-Dihydropyridazine-4-Carboxylic Acids. Molecules 2009, 14, 68–77. [Google Scholar]
- Al-Matar, H.M.; Khalil, K.D.; Al-Kanderi, M.F.; Elnagdi, M.H. Studies on 3-oxoalkanenitriles: novel rearrangement reactions observed in studies of the chemistry of 3-heteroaroyl-3-oxoalkanenitriles as novel routes to 2-dialkylaminopyridines. Molecules 2012, 17, 897–909. [Google Scholar] [CrossRef]
- Tomczak, E. Application of ANN and EA for description of metal ions sorption on chitosan foamed structure—Equilibrium and dynamics of packed column. Comp. Chem. Eng. 2011, 35, 226–235. [Google Scholar] [CrossRef]
- Kaupp, G.; Naimi-Jamal, M.R.; Schmeyers, J. Uncatalysed Knoevenagel condensation in aqueous medium at room temperature. Tetrahedron 2003, 59, 3753–3760. [Google Scholar] [CrossRef]
- Babu, N.S.; Pasha, N.; Venkateswara-Rao, K.T.; Sai-Prasad, P.S.; Lingaiah, N. A heterogeneous strong basic Mg/La mixed oxide catalyst for efficient synthesis of polyfunctionalized pyrans. Tetrahedron Lett. 2008, 49, 2730–2733. [Google Scholar] [CrossRef]
- Elagami, A.A.; Sewilam, S.Z.; El-Taweel, F.M.A.; Elnagdi, M.H. Nitriles in heterocyclic synthesis: Novel synthesis of benzo[b]pyrans naphtho[1,2-b]pyrano, naphtho[2,1-b]pyrans, pyrano[3,2-b]quinolines and pyrano[3,2-c] quinolines. Collect. Czech. Chem. Commun. 1988, 53, 1534. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 2, 3, 4, 7, 11, 15a,b, 17a,b. are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Khalil, K.D.; Al-Matar, H.M. Chitosan Based Heterogeneous Catalyses: Chitosan-Grafted-Poly(4-Vinylpyridne) as an Efficient Catalyst for Michael Additions and Alkylpyridazinyl Carbonitrile Oxidation. Molecules 2013, 18, 5288-5305. https://doi.org/10.3390/molecules18055288
Khalil KD, Al-Matar HM. Chitosan Based Heterogeneous Catalyses: Chitosan-Grafted-Poly(4-Vinylpyridne) as an Efficient Catalyst for Michael Additions and Alkylpyridazinyl Carbonitrile Oxidation. Molecules. 2013; 18(5):5288-5305. https://doi.org/10.3390/molecules18055288
Chicago/Turabian StyleKhalil, Khaled D., and Hamad M. Al-Matar. 2013. "Chitosan Based Heterogeneous Catalyses: Chitosan-Grafted-Poly(4-Vinylpyridne) as an Efficient Catalyst for Michael Additions and Alkylpyridazinyl Carbonitrile Oxidation" Molecules 18, no. 5: 5288-5305. https://doi.org/10.3390/molecules18055288
APA StyleKhalil, K. D., & Al-Matar, H. M. (2013). Chitosan Based Heterogeneous Catalyses: Chitosan-Grafted-Poly(4-Vinylpyridne) as an Efficient Catalyst for Michael Additions and Alkylpyridazinyl Carbonitrile Oxidation. Molecules, 18(5), 5288-5305. https://doi.org/10.3390/molecules18055288