Deciphering the Role of Phytoalexins in Plant-Microorganism Interactions and Human Health
Abstract
:1. Phytoalexins: A Global Survey
- ○
- Chemical diversity
- ○
- Main biosynthetic pathways and regulation networks
- ○
- Biological activity against microorganisms
- ○
- Molecular engineering for disease resistance in plants
- ○
- Metabolism/Transport in fungi
- ○
- Role in human health
2. Chemical Diversity of Phytoalexins
| Plant Families (in Alphabetical Order) | Types of Phytoalexins/Examples | References |
|---|---|---|
| Amaryllidaceae | Flavans | [19] |
| Brassicaceae (Cruciferae) | Indole phytoalexins/camalexin | [20] |
| Sulfur-containing phytoalexins/brassinin | [21] | |
| Chenopodiaceae | Flavanones/betagarin Isoflavones/betavulgarin | [22] |
| Compositae | Polyacetylenes/safynol | [23] |
| Convolvulaceae | Furanosesquiterpenes/Ipomeamarone | [24] |
| Euphorbiaceae | Diterpenes/casbene | [25] |
| Poaceae | Diterpenoids:Momilactones; Oryzalexins; Zealexins; Phytocassanes; Kauralexins | [8,26] |
| Deoxyanthocyanidins/luteolinidin and apigeninidin | [26,27] | |
| Flavanones/sakuranetin | [1] | |
| Phenylamides | [28] | |
| Leguminosae | Isoflavones Isoflavanones Isoflavans Coumestans Pterocarpans/pisatin, phaseollin, glyceollin and maiackiain Furanoacetylenes/wyerone Stilbenes/resveratrol Pterocarpens | [1] and references therein |
| Linaceae | Phenylpropanoids/coniferyl alcohol | [29] |
| Malvaceae | Terpenoids naphtaldehydes/gossypol | [11] |
| Moraceae | Furanopterocarpans/moracins A-H | [30] |
| Orchidaceae | Dihydrophenanthrenes/loroglossol | [31] |
| Rutaceae | Methylated phenolic compounds/xanthoxylin | [32] |
| Umbelliferae | Polyacetylenes/falcarinol | [33] |
| Phenolics: xanthotoxin | [34] | |
| 6-methoxymellein | [35] | |
| Vitaceae | Stilbenes/resveratrol | [9] |
| Rosaceae | Biphenyls/auarperin | [36] |
| Dibenzofurans/cotonefurans | ||
| Solanaceae | Phenylpropanoid related compounds | [1] and references therein |
| Steroid glycoalkaloids | ||
| Norsequi and sesquiterpenoids | ||
| Coumarins | ||
| Polyacetylenic derivatives |
3. Main Biosynthetic Pathways
- (i)
- The phenylpropanoic-polymalonic acid route
- (ii)
- The methylerythritol phosphate and geranyl-geranyl diphosphate pathway
- (iii)
- The indole phytoalexin pathway
3.1. Phytoalexins Deriving from the Phenylpropanoic-Polymalonic Acid Route
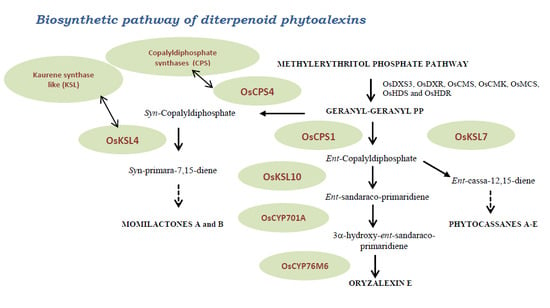
3.2. Mevalonoid-Derived Phytoalexins


3.3. Indole Phytoalexins
4. Regulation Networks

5. Biological Activity against Microorganisms
6. Engineering of Phytoalexins and Role in Plant Defense Mechanisms
7. Fungal Metabolism and Transporters
8. Role of Phytoalexins in Human Health
9. Concluding Remarks
Abbreviation
| IFS | 2-hydroxy isoflavanone synthase; |
| DMI | 7,2'-dihydroxy-4'-methoxy-isoflavanol; |
| DMDI | 7,2'-dihydroxy-4',5'-methylenedioxy-isoflavanol; |
| HMM | 6α-hydroxymaackiain 3-O-methyltransferase |
| HI4'OMT: SAM: | 2,7,4'-trihydroxy-isoflavanone 4'-O-methyltransferase; |
| STS | stilbene synthase; |
| CHS | chalcone synthase. |
Author Contributions
Conflicts of Interest
References
- Jeandet, P.; Clément, C.; Courot, E.; Cordelier, S. Modulation of phytoalexin biosynthesis in engineered plants for disease resistance. Int. J. Mol. Sci. 2013, 14, 14136–14170. [Google Scholar] [PubMed]
- Ahuja, I.; Kissen, R.; Bones, A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012, 17, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.O.; Börger, H. Experimentelle Untersuchungen über die Phytophthora Resistenz der Kartoffel. Arb. Biol. Reichsanst. Land Forstwirtsch. 1940, 23, 189–231. [Google Scholar]
- Deverall, B.J. Introduction. In Phytoalexins; Bailey, J.A., Mansfield, J.W., Eds.; Blackie: Glasgow/London, UK, 1982; pp. 1–20. [Google Scholar]
- Ingham, J.L. Phytoalexins from the Leguminosae. In Phytoalexins; Bailey, J.A., Mansfield, J.W., Eds.; Blackie: Glasgow/London, UK, 1982; pp. 21–80. [Google Scholar]
- Kuc, J. Phytoalexins from the Solanaceae. In Phytoalexins; Bailey, J.A., Mansfield, J.W., Eds.; Blackie: Glasgow/London, UK, 1982; pp. 81–105. [Google Scholar]
- Coxon, D.T. Phytoalexins from other plant families. In Phytoalexins; Bailey, J.A., Mansfield, J.W., Eds.; Blackie: Glasgow/London, UK, 1982; pp. 106–132. [Google Scholar]
- Schmelz, E.A.; Huffaker, A.; Sims, J.W.; Christensen, S.A.; Lu, X.; Okada, K.; Peters, R.J. Biosynthesis, elicitation and roles of monocot terpenoid phytoalexins. Plant J. 2014, 79, 659–678. [Google Scholar] [CrossRef] [PubMed]
- Langcake, P.; Pryce, R.J. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- Jeandet, P.; Delaunois, B.; Conreux, A.; Donnez, D.; Nuzzo, V.; Cordelier, S.; Clément, C.; Courot, E. Biosynthesis, metabolism, molecular engineering and biological functions of stilbene phytoalexins in plants. BioFactors 2010, 36, 331–341. [Google Scholar] [CrossRef]
- Sunilkumar, G.; Campbell, L.M.; Pukhaber, L.; Stipanovic, R.D.; Rathore, K.S. Engineering cottonseed for use in human nutrition by tissue-specific reduction of toxic gossypol. Proc. Natl. Acad. Sci. USA 2006, 103, 18054–18059. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Liu, Y.; Yang, K.Y.; Han, L.; Mao, G.; Glazebrook, J.; Zhang, S. A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis. Proc. Natl. Acad. Sci. USA 2008, 105, 5638–5643. [Google Scholar] [CrossRef] [PubMed]
- Delaunois, B.; Cordelier, S.; Conreux, A.; Clément, C.; Jeandet, P. Molecular engineering of resveratrol in plants. Plant Biotechnol. J. 2009, 7, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Delaunois, B.; Aziz, A.; Donnez, D.; Vasserot, Y.; Cordelier, S.; Courot, E. Metabolic engineering of yeast and plants for the production of the biologically active hydroxystilbene, resveratrol. J. Biomed. Biotechnol. 2012. [Google Scholar] [CrossRef]
- Mansfield, J.W.; Bailey, J.A. Phytoalexins: Current problems and future prospects. In Phytoalexins; Bailey, J.A., Mansfield, J.W., Eds.; Blackie: Glasgow/London, UK, 1982; pp. 319–323. [Google Scholar]
- Hammerschmidt, R. Phytoalexins: What have we learned after 60 years? Annu. Rev. Phytopathol. 1999, 37, 285–306. [Google Scholar] [CrossRef]
- Wu, Q.; VanEtten, H.D. Introduction of plant and fungal genes into pea (Pisum sativum L.) hairy roots reduces their ability to produce pisatin and affects their response to a fungal pathogen. Mol. Plant-Microbe Interact. 2004, 17, 798–804. [Google Scholar] [CrossRef]
- Cruickshank, I.A.M.; Perrin, D.R. Isolation of a phytoalexin from Pisum sativum L. Nature 1960, 187, 799–800. [Google Scholar] [CrossRef] [PubMed]
- Coxon, D.T.; O’Neill, T.M.; Mansfield, J.W.; Porter, A.E.A. Identification of three hydroxyflavan phytoalexins from daffodil bulbs. Phytochemistry 1980, 19, 889–891. [Google Scholar] [CrossRef]
- Browne, L.M.; Conn, K.L.; Ayert, W.A.; Tewari, J.P. The camalexins: New phytoalexins produced in the leaves of Camelia sativa (Cruciferae). Tetrahedron 1991, 47, 3909–3914. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Okanga, F.I.; Zaharia, I.L.; Khan, A.G. Phytoalexins from crucifers: Synthesis, biosynthesis and biotransformation. Phytochemistry 2000, 53, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Geigert, J.; Stermitz, F.R.; Johnson, G.; Maag, D.D.; Johnson, D.K. Two phytoalexins from sugarbeet (Beta vulgaris) leaves. Tetrahedron 1973, 29, 2703–2706. [Google Scholar] [CrossRef]
- Allen, E.H.; Thomas, C.A. Trans-trans-3,11-tridecadiene-5,7,9-triyne-1,2-diol, an antifungal polyacetylene from diseased safflower (Carthamus tinctorius). Phytochemistry 1971, 10, 1579–1582. [Google Scholar] [CrossRef]
- Uritani, I.; Uritani, M.; Yamada, H. Similar metabolic alterations induced in sweet potato by poisonous chemicals and by Ceratostomella fimbriata. Phytopathology 1960, 50, 30–34. [Google Scholar]
- Sitton, D.; West, C.A. Casbene: An antifungal diterpene produced in cell-free extracts of Ricinus communis seedlings. Phytochemistry 1975, 14, 1921–1925. [Google Scholar] [CrossRef]
- Poloni, A.; Schirawski, J. Red card for pathogens: Phytoalexins in sorghum and maize. Molecules 2014, 19, 9114–9133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, S.C.; de Verdier, K.; Nicholson, R. Accumulation of 3-deoxyanthocyanidin phytoalexins and resistance to Colletotrichum sublineolum in sorghum. Physiol. Mol. Plant Pathol. 1999, 55, 263–273. [Google Scholar] [CrossRef]
- Lin Park, H.; Lee, S.W.; Jung, K.H.; Hahn, T.R.; Cho, M.H. Transcriptomic analysis of UV-treated rice leaves reveals UV-induced phytoalexin biosynthetic pathways and their regulatory networks in rice. Phytochemistry 2013, 96, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Keen, N.T.; Littlefield, L.J. The possible association of phytoalexins with resistant gene expression in flax to Melamspora lini. Physiol. Plant Pathol. 1975, 14, 275–280. [Google Scholar]
- Takasugi, M.; Nagao, S.; Masamune, T.; Shirata, A.; Takahashi, K. Structures of moracins E, F, G and H, new phytoalexins from diseased mulberry. Tetrahedron Lett. 1979, 28, 4675–4678. [Google Scholar] [CrossRef]
- Ward, E.W.B.; Unwin, C.H.; Stoessel, A. Loroglossol: An orchid phytoalexin. Phytopathology 1975, 65, 632–633. [Google Scholar] [CrossRef]
- Hartmann, G.; Nienhaus, F. The isolation of xanthoxylin from the bark of Phytophthora- and Hendersonula-infected Citrus lemon and its fungitoxic effect. Phytopathol. Z. 1974, 81, 97–113. [Google Scholar] [CrossRef]
- Harding, V.K.; Heale, J.B. The accumulation of inhibitory compounds in the induced resistance response of carrot root slices to Botrytis cinerea. Physiol. Plant Pathol. 1981, 18, 7–15. [Google Scholar] [CrossRef]
- Johnson, C.; Brannon, D.R.; Kuc, J. Xanthotoxin: A phytoalexin of Pastinaca sativa root. Phytochemistry 1973, 12, 2961–2962. [Google Scholar] [CrossRef]
- Condon, P.; Kuc, J.; Draudt, H.N. Production of 3-methyl-6-methoxy-8-hydroxy-3,4-dihydroisocoumarin by carrot root tissue. Phytopathology 1963, 53, 1244–1250. [Google Scholar]
- Kokubun, T.; Harborne, J.B. Phytoalexin induction in the sapwood of plants of the Maloideae (Rosaceae): Biphenyls or dibenzofurans. Phytochemistry 1995, 40, 1649–1654. [Google Scholar] [CrossRef]
- Chen, Y.; Tao, Y.; Gao, C.; Cao, W.; Huang, R. Natural products from the genus Tephrosia. Molecules 2014, 19, 1432–1458. [Google Scholar] [CrossRef] [PubMed]
- Deavours, B.E.; Dixon, R.A. Metabolic engineering of isoflavonoid biosynthesis in alfalfa. Plant Physiol. 2005, 138, 2245–2259. [Google Scholar] [CrossRef] [PubMed]
- Kaimoyo, E.; VanEtten, H.D. Inactivation of pea genes by RNAi supports the involvement of two similar O-methyltransferases in the biosynthesis of (+)-pisatin and of chiral intermediates with a configuration opposite that found in (+)-pisatin. Phytochemistry 2008, 69, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Thomma, B.P.H.J.; Nelissen, I.; Eggermont, K.; Broekaert, W.F. Deficiency in phytoalexin production causes enhanced susceptibilty of Arabidopsis thaliana to the fungus Alternaria brassicicola. Plant J. 1999, 19, 163–117. [Google Scholar] [CrossRef] [PubMed]
- Van Wees, S.C.; Chang, H.S.; Zhu, T.; Glazebrook, J. Characterization of the early response of Arabidopsis to Alternaria brassicicola infection using expression profiling. Plant Physiol. 2003, 132, 606–617. [Google Scholar] [CrossRef] [PubMed]
- Rowe, H.C.; Walley, J.W.; Corwin, J.; Chan, E.K.F.; Dehesh, K.; Kliebenstein, D.J. Deficiencies in jasmonate-mediated plant defense reveal quantitative variation in Botrytis cinerea pathogenesis. PLoS Pathog. 2010, 6, e1000861. [Google Scholar] [CrossRef] [PubMed]
- Riemann, M.; Haga, K.; Shimizu, T.; Okada, K.; Ando, S.; Mochizuki, S.; Nishizawa, Y.; Yamanouchi, U.; Nick, P.; Yano, M.; et al. Identification of rice Allene Oxide Cyclase mutants and the function of jasmonate for defence against Magnaporthe oryzae. Plant J. 2013, 74, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Nawrath, C.; Métraux, J.P. Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 1999, 11, 1393–1404. [Google Scholar] [PubMed]
- Roetschi, A.; Si-Ammour, A.; Belbahri, L.; Mauch, F.; Mauch-Mani, B. Characterization of an Arabidopsis-Phytophthora pathosystem: Resistance requires a functional PAD2 gene and is independent of salicylic acid, ethylene and jasmonic acid signalling. Plant J. 2001, 28, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Denby, K.J.; Jason, L.J.M.; Murray, S.L.; Last, R.L. ups1, an Arabidopsis thaliana camalexin accumulation mutant defective in multiple defence signalling pathways. Plant J. 2005, 41, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Heck, S.; Grau, T.; Buchala, A.; Métraux, J.P.; Nawrath, C. Genetic evidence that expression of NahG modifies defence pathways independent of salicylic acid biosynthesis in the Arabidopsis-Pseudomonas syringae pv. tomato interaction. Plant J. 2003, 36, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Robert-Seilaniantz, A.; MacLean, D.; Jikumaru, Y.; Hill, L.; Yamaguchi, S.; Kamiya, Y.; Jones, J.D.G. The microRNA miR393 re-directs secondary metabolite biosynthesis away from camalexin and towards glucosinates. Plant J. 2011, 67, 218–231. [Google Scholar] [CrossRef] [PubMed]
- Goosens, J.F.V.; Vendrig, J.C. Effects of abscissic acid, cytokinins, and light on isoflavonoid phytoalexin accumulation in Phaseolus vulgaris. Planta 1982, 154, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Ward, E.W.; Cahill, D.M.; Bhattacharyya, M.K. Abscisic acid suppression of phenylalanine ammonia-lyase activity and mRNA, and resistance of soybeans to Phytophthora megasperma f.s.p. glycinea. Plant Physiol. 1989, 91, 23–27. [Google Scholar] [CrossRef]
- Mohr, P.; Cahill, D.M. Relative roles of glyceollin, lignin and the hypersensitive response and the influence of ABA in compatible and incompatible interactions of soybeans with Phytophthora sojae. Physiol. Mol. Plant Pathol. 2001, 58, 31–41. [Google Scholar] [CrossRef]
- Henfling, J.W.D.M.; Bostock, R.M.; Kuc, J. Effect of abscisic acid on rishitin and lubimin accumulation and resistance to Phytophthora infestans and Cladosporium cucumerinum in potato tuber tissue slices. Phytopathology 1980, 70, 1074–1078. [Google Scholar] [CrossRef]
- Mialoundama, A.S.; Heintz, D.; Debayle, D.; Rahier, A.; Camara, B.; Bouvier, F. Abscisic acid negatively regulates elicitor-induced synthesis of capsidiol in wild tobacco. Plant Physiol. 2009, 150, 1556–1566. [Google Scholar] [CrossRef] [PubMed]
- Grosskinsky, D.K.; Naseem, M.; Abdelmoshem, U.A.; Plickert, N.; Engelke, T.; Griebel, T.; Zeier, J.; Novak, O.; Strand, M.; Pfeifhofer, H.; et al. Cytokinins mediate resistance against Pseudomonas syringae in tobacco through increased antimicrobial phytoalexin synthesis independent of salicylic acid signaling. Plant Physiol. 2011, 157, 815–830. [Google Scholar] [CrossRef] [PubMed]
- Kurusu, T.; Hamada, J.; Nokajima, H.; Kitagawa, Y.; Kiyoduka, M.; Takahashi, A.; Hanamata, S.; Ohno, R.; Hayashi, T.; Okada, K.; et al. Regulation of microbe-associated molecular pattern-induced hypersensitive cell death, phytoalexin production, and defense gene expression by calcineurin B-like protein-interacting protein kinases, OsCIPK14/15, in rice cultured cells. Plant Physiol. 2010, 153, 678–692. [Google Scholar] [CrossRef] [PubMed]
- Ono, E.; Wong, H.L.; Kawasaki, T.; Hasegawa, M.; Kodama, O.; Shimamoto, K. Essential role of the small GTPase Rac in disease resistance of rice. Proc. Natl. Acad. Sci. USA 2001, 98, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Sadawa, K.; Hasegawa, M.; Tokuda, L.; Kameyama, J.; Kodama, O.; Kohchi, T.; Yoshida, K.; Shinmyo, A. Enhanced resistance to blast fungus and bacterial blight in transgenic rice constitutively expressing OsSBP, a rice homologue of mamalian selenium-binding proteins. Biosci. Biotechnol. Biochem. 2004, 68, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Rauhut, T.; Luberacki, B.; Seitz, H.U.; Glawischnig, E. Inducible expression of a Nep1-like protein serves as a model trigger system of camalexin biosynthesis. Phytochemistry 2009, 70, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Formela, M.; Samardakiewicz, S.; Marczak, L.; Nowak, W.; Narozna, D.; Waldemar, B.; Kasprowicz-Maluski, A.; Morkunas, I. Effects of endogenous signals and Fusarium oxysporum on the mechanism regulating genistein synthesis and accumulation in yellow lupine and their impact on plant cell cytoskeleton. Molecules 2014, 19, 13392–13421. [Google Scholar] [CrossRef] [PubMed]
- Parkhi, V.; Kumar, V.; Campbell, L.M.; Bell, A.A.; Shah, J.; Rathore, K.S. Resistance against various fungal pathogens and reniform nematode in transgenic cotton plants expressing Arabidopsis NRP1. Transgenic Res. 2010, 19, 959–975. [Google Scholar] [CrossRef] [PubMed]
- Rathmell, W.G.; Smith, D.A. Lack of activity of selected isoflavonoid phytoalexins as protectant fungicides. Pestic. Sci. 1980, 11, 568–572. [Google Scholar] [CrossRef]
- Ingham, J.L.; Deverall, B.J.; Kuc, J.; Coxon, D.T.; Stoessl, A.; VanEtten, H.D.; Matthews, D.E.; Smith, D.A. Toxicity of phytoalexins. In Phytoalexins; Bailey, J.A., Mansfield, J.W., Eds.; Blackie: Glasgow/London, UK, 1982; pp. 218–252. [Google Scholar]
- Adrian, M.; Jeandet, P.; Veneau, J.; Weston, L.A.; Bessis, R. Biological activity of resveratrol, a stilbenic compound from grapevines, against Botrytis cinerea, the causal agent for gray mold. J. Chem. Ecol. 1997, 23, 1689–1702. [Google Scholar] [CrossRef]
- Adrian, M.; Jeandet, P. Effects of resveratrol on the ultrastructure of Botrytis cinerea conidia and biological significance in plant/pathogen interactions. Fitoterapia 2012, 83, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Mitsuhara, I.; Seo, S.; Okada, K.; Yamane, H.; Iwai, T.; Ohashi, Y. Analysis on blast fungus-responsive characters of a flavonoid phytoalexin sakuranetin; Accumulation in infected rice leaves, antifungal activity and detoxification by fungus. Molecules 2014, 19, 11404–11418. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.E.; Dennis, C. The effect of post-infectional potato tuber metabolites and surfactants on zoospores of Oomycetes. Physiol. Plant Pathol. 1977, 11, 163–169. [Google Scholar] [CrossRef]
- Pezet, R.; Pont, V. Ultrastructural observations of pterostilbene fungitoxicity in dormant conidia of Botrytis cinerea Pers. J. Phytopathol. 1990, 129, 29–30. [Google Scholar] [CrossRef]
- VanEtten, H.D.; Bateman, D.F. Studies on the mode of action of the phytoalexin phaseollin. Phytopathology 1971, 61, 1363–1372. [Google Scholar] [CrossRef]
- Rossall, S.; Mansfield, J.W.; Huston, R.A. Death of Botrytis cinerea and B. fabae following exposure to wyerone derivatives in vitro and during infection development in broad bean leaves. Physiol. Plant Pathol. 1980, 16, 135–146. [Google Scholar] [CrossRef]
- Woods, J.A.; Hafield, J.A.; Pettit, G.R.; Fox, B.W.; McGown, A.T. The interaction with tubulin of a series of stilbenes based on combretastatin A-4. Br. J. Cancer 1995, 71, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Shlezinger, N.; Minz, A.; Gur, Y.; Hatam, I.; Dagdas, Y.F.; Talbot, N.J.; Sharon, A. Anti-apoptotic machinery protects the necrotrophic fungus Botrytis cinerea from host-induced apoptotic-like cell death during plant infection. PLoS Pathog. 2011, 7, e1002185. [Google Scholar] [CrossRef] [PubMed]
- Sanzani, S.; Schena, L.; Ippolito, A. Effectiveness of phenolic compounds against citrus green mould. Molecules 2014, 19, 12500–12508. [Google Scholar] [CrossRef] [PubMed]
- Sanzani, S.M.; de Girolamo, A.; Schena, L.; Solfrizzo, M.; Ippolito, A.; Visconti, A. Control of Penicillium expansum and patulin accumulation on apples by quercetin and umbelliferone. Eur. Food Res. Technol. 2009, 228, 381–389. [Google Scholar] [CrossRef]
- Pont, V.; Pezet, R. Relation between the chemical structure and biological activity of hydroxystilbenes against Botrytis cinerea. J. Phytopathol. 1990, 130, 1–8. [Google Scholar] [CrossRef]
- Chalal, M.; Klinguer, A.; Echairi, A.; Meunier, P.; Vervandier-Fasseur, D.; Adrian, M. Antimicrobial activity of resveratrol analogues. Molecules 2014, 19, 7679–7688. [Google Scholar] [CrossRef] [PubMed]
- Lyon, G.D.; Bayliss, C.E. The effect of rishitin on Erwinia carotovora var. atroseptica and other bacteria. Physiol. Plant Pathol. 1975, 6, 177–186. [Google Scholar] [CrossRef]
- Hain, R.; Reif, H.J.; Krause, E.; Langebartels, R.; Kindl, H.; Vornam, B.; Wiese, W.; Schmelzer, E.; Schreier, P.; Stöcker, R.; et al. Disease resistance results from foreign phytoalexin expression in a novel plant. Nature 1993, 361, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Gachon, C.; Baltz, R.; Saindrenan, P. Over-expression of a scopoletin glucosyltransferase in Nicotiana tabacum leads to precocious lesion formation during the hypersensitive response to tobacco mosaic virus but does not affect virus resistance. Plant Mol. Biol. 2004, 54, 137–146. [Google Scholar] [CrossRef] [PubMed]
- He, X.Z.; Dixon, R.A. Genetic manipulation of isoflavone 7-O-methyltransferase enhances biosynthesis of 4'-O-methylated isoflavonoid phytoalexins and disease resistance in alfalfa. Plant Cell 2000, 12, 1689–1702. [Google Scholar] [CrossRef] [PubMed]
- Schmidlin, L.; Poutaraud, A.; Claudel, P.; Mestre, P.; Prado, E.; Santos-Rosa, M.; Wiedemann-Merdinoglu, S.; Karst, F.; Merdinoglu, D.; Hugueney, P. A stress-inducible resveratrol O-methyltransferase involved in the biosynthesis of pterostilbene in grapevine. Plant Physiol. 2008, 148, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Zernova, O.V.; Lygin, A.V.; Pawlowski, M.L.; Hill, C.B.; Hartman, G.L.; Widholm, J.M.; Lozovaya, V.V. Regulation of plant immunity through modulation of phytoalexin synthesis. Molecules 2014, 19, 7480–7496. [Google Scholar] [CrossRef] [PubMed]
- Graham, T.L.; Graham, M.Y.; Subramanian, S.; Yu, O. RNAi silencing of genes for elicitation or biosynthesis of 5-deoxyisoflavonoids suppresses race-specific resistance and hypersensitive cell death in Phytophthora sojae infected tissues. Plant Physiol. 2007, 144, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Ibraheem, F.; Gaffoor, I.; Chopra, S. Flavonoid phytoalexin-dependent resistance to anthracnose leaf blight requires a functional yellow seed1 in Sorghum bicolor. Genetics 2010, 184, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Baltz, R.; Schmitt, C.; Beffa, R.; Fritig, B.; Saindrenan, P. Downregulation of a pathogen-responsive tobacco UDP-Glc:phenylpropanoid glucosyltransferase reduces scopoletin glucoside accumulation, enhances oxidative stress, and weakens virus resistance. Plant Cell 2002, 14, 1093–1107. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Ohm, R.A.; Devappa, R.; Lee, H.B.; Grigoriev, I.V.; Kim, B.Y.; Ahn, J.S. Transcriptional responses of the bdtf1-deletion mutant to the phytoalexin brassinin in the necrotrophic fungus Alternaria brassicicola. Molecules 2014, 19, 10717–10732. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.C.; Ahiahonu, P.W.K.; Hossain, M. Detoxification of the cruciferous phytoalexin brassinin in Sclerotinia sclerotiorum requires an inducible glucosyltransferase. Phytochemistry 2004, 65, 2685–2694. [Google Scholar] [CrossRef] [PubMed]
- Delserone, L.M.; McCluskey, K.; Matthews, D.E.; VanEtten, H.D. Pisatin demethylation by fungal pathogens and non pathogens on pea: Association with pisatin tolerance and virulence. Physiol. Mol. Plant Pathol. 1999, 55, 317–326. [Google Scholar] [CrossRef]
- Li, D.; Chung, K.R.; Smith, D.A.; Schardl, C.L. The Fusarium solani gene encoding kievitone hydratase, a secreted enzyme that catalyzes detoxification of a bean phytoalexin. Mol. Plant-Microbe Interact. 1995, 8, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.C.; Gadagi, R.S.; Jha, M.; Sarma-Mamillapalle, V.K. Detoxification of the phytoalexin brassinin by isolates of Leptosphaeria maculans pathogenic on brown mustard involves an inducible hydrolase. Phytochemistry 2007, 68, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Pedras, M.S.C.; Minic, Z.; Jha, M. Brassinin oxidase, a fungal detoxifying enzyme to overcome a plant defense—purification, characterization and inhibition. FEBS J. 2008, 275, 3691–3705. [Google Scholar] [CrossRef] [PubMed]
- Pezet, R. Purification and characterization of a 32-kDa laccase-like stilbene oxidase produced by Botrytis cinerea Pers.: Fr. FEMS Microbiol. Lett. 1998, 167, 203–208. [Google Scholar] [CrossRef]
- Breuil, A.C.; Adrian, M.; Pirio, N.; Meunier, P.; Bessis, R.; Jeandet, P. Metabolism of stilbene phytoalexins by Botrytis cinerea: Characterization of a resveratrol dehydrodimer. Tetrahedron Lett. 1998, 39, 537–540. [Google Scholar] [CrossRef]
- Breuil, A.C.; Jeandet, P.; Chopin, F.; Adrian, M.; Pirio, N.; Meunier, P.; Bessis, R. Characterization of a pterostilbene dehydrodimer produced by laccase of Botrytis cinerea. Phytopathology 1999, 89, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Sbaghi, M.; Jeandet, P.; Bessis, R.; Leroux, P. Metabolism of stilbene type-phytoalexins in relation to the pathogenicity of Botrytis cinerea to grapevines. Plant Pathol. 1996, 45, 139–144. [Google Scholar] [CrossRef]
- Hammerbacher, A.; Schmidt, A.; Wadke, N.; Wright, L.P.; Schneider, B.; Bohlmann, J.; Brand, W.A.; Fenning, T.M.; Gershenzon, J.; Paetz, C. A common fungal associate of the spruce bark beetle metabolizes the stilbene defenses of Norway spruce. Plant Physiol. 2013, 162, 1324–1336. [Google Scholar] [CrossRef] [PubMed]
- Del Sorbo, G.; Schoonbeek, H.J.; de Waard, M.A. Fungal transporters involved in efflux of natural toxic compounds and fungicides. Fungal Genet. Biol. 2000, 30, 1–15. [Google Scholar] [CrossRef] [PubMed]
- De Ward, M.A.; Andrade, A.C.; Hayashi, K.; Schoonbeek, H.J.; Stergiopoulos, I.; Zwiers, L.H. Impact of fungal drug transporters on fungicide sensitivity, multidrug resistance and virulence. Pest Manag. Sci. 2006, 62, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.J.; Mylonakis, E. Efflux in fungi: La pièce de résistance. PLoS Pathog. 2009, 5, e1000486. [Google Scholar] [CrossRef] [PubMed]
- Urban, M.; Bhargava, T.; Hamer, J.E. An ATP-driven efflux pump is a novel pathogenicity factor in rice blast disease. EMBO J. 1999, 18, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Schoonbeek, H.J.; del Sorbo, G.; de Waard, M.A. The ABC transporter BcatrB affects the sensitivity of Botrytis cinerea to the phytoalexin resveratrol and the fungicide fenpiclonil. Mol. Plant-Microbe Interact. 2001, 14, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Fleissner, A.; Sopalla, C.; Weltring, K.M. An ATP-binding cassette multidrug-resistance transporter is necessary for tolerance of Gibberella pulicaris to phytoalexins and virulence on potato tubers. Mol. Plant-Microbe Interact. 2002, 15, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Del Sorbo, G.; Ruocco, M.; Schoonbeek, H.J.; Scala, F.; Pane, C.; Vinale, F.; de Waard, M.A. Cloning and functional characterization of BcatrA, a gene encoding an ABC transporter of the plant pathogenic fungus Botryotinia fuckeliana (Botrytis cinerea). Mycol. Res. 2008, 112, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Stefanato, F.L.; Abou-Mansour, E.; Buchala, A.; Kretschmer, M.; Mosbach, A.; Hahn, M.; Bochet, C.G.; Métraux, J.P.; Schoonbeek, H.J. The ABC transporter BcatrB from Botrytis cinerea exports camalexin and is a virulence factor on Arabidopsis thaliana. Plant J. 2009, 58, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Coleman, J.J.; White, G.J.; Rodriguez-Carres, M.; VanEtten, H.D. An ABC transporter and a cytochrome P450 of Nectria haematococca MPVI are virulence factors on pea and are the major tolerance mechanisms to the phytoalexin pisatin. Mol. Plant-Microbe Interact. 2010, 24, 368–376. [Google Scholar] [CrossRef]
- Zwiers, L.H.; de Waard, M.A. Characterization of the ABC transporter genes MgAtr1 and MgAtr2 from the wheat pathogen Mycosphaerella graminicola. Fungal Genet. Biol. 2000, 30, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Stergiopoulos, I.; van Nistelrooy, J.G.M.; Kema, G.H.J.; de Waard, M.A. Multiple mechanisms account for variation in base-line sensitivity to azole fungicides in field isolates of Mycosphaerella graminicola. Pest Manag. Sci. 2003, 59, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Schoonbeek, H.J.; van Nistelrooy, J.G.M.; de Waard, M.A. Functional analysis of ABC transporter genes from Botrytis cinerea identifies BcatrB as a transporter of eugenol. Eur. J. Plant Pathol. 2003, 109, 1003–1011. [Google Scholar] [CrossRef]
- Schoonbeek, H.J.; Raaijmakers, J.M.; de Waard, M.A. Fungal ABC transporters and microbial interactions in natural environments. Mol. Plant-Microbe Interact. 2002, 15, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, T.; Schoonbeek, H.J.; de Waard, M.A. The ABC transporter BcatrB from Botrytis cinereais a determinant of the activity of the phenylpyrrole fungicide fludioxonil. Pest Manag. Sci. 2001, 57, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.C.; del Sorbo, G.; van Nistelrooy, J.G.M.; de Waard, M.A. The ABC transporter AtrB from Aspergillus nidulans mediates resistance to all major classes of fungicides and some natural toxic compounds. Microbiology 2000, 146, 1987–1997. [Google Scholar] [PubMed]
- Zwiers, L.H.; Stergiopoulos, I.; Gielkens, M.M.C.; Goodall, S.D.; de Waard, M.A. ABC transporters of the wheat pathogen Mycosphaerella graminicola function as protectants against biotic and xenobiotic toxic compounds. Mol. Genet. Genomics 2003, 269, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Nakaune, R.; Hamamoto, H.; Imada, J.; Akutsu, K.; Hibi, T. A novel ABC transporter gene, PMR5, is involved in multidrug resistance in the phytopathogenic fungus Penicillium digitatum. Mol. Genet. Genomics 2002, 267, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Mena, G.; Sanchez-Gonzalez, M.; Juan, M.E.; Planas, J.M. Maslinic acid, a natural phytoalexin-type triterpene from olives—A promising nutraceutical? Molecules 2014, 19, 11538–11559. [Google Scholar] [CrossRef]
- Jahangir, M.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Health-affecting compounds in Brassicaceae. Compr. Rev. Food Sci. Food Saf. 2009, 8, 31–43. [Google Scholar] [CrossRef]
- Smith, B.; Randle, D.; Mezencev, R.; Thomas, L.; Hinton, C.; Odero-Marah, V. Camalexin-induced apoptosis in prostate cancer cells involves alterations of expression and activity of lysosomal protease cathepsin D. Molecules 2014, 19, 3988–4005. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Browning, J.D.; Awika, J.M. Sorghum 3-deoxyanthocyanidins possess strong phase II enzyme inducer activity and cancer cell growth inhibition properties. J. Agric. Food Chem. 2009, 57, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Kello, M.; Drutovic, D.; Chripkova, M.; Pilatova, M.; Budovska, L.; Kulikova, L.; Urdzik, P.; Mojzis, J. ROS-dependent antiproliferative effect of brassinin derivative homobrassinin in human colorectal cancer Caco2 cells. Molecules 2014, 19, 10877–10897. [Google Scholar] [CrossRef] [PubMed]
- Athar, M.; Back, J.H.; Tang, X.; Kim, K.H.; Kopelovich, L.; Bickers, D.R.; Kim, A.L. Resveratrol: A review of preclinical studies for human cancer protection. Toxicol. Appl. Pharmacol. 2007, 224, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Metha, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Fuda, S.; Debatin, K.M. Sensitization for tumor necrosis factor-related apotosis-inducing ligand-induced apoptosis by the chemopreventive agent resveratrol. Cancer Res. 2004, 64, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Budovska, M.; Pilatova, M.; Varinska, L.; Mojzis, J.; Mezncev, R. The synthesis and anticancer activity of analogs of the indole phytoalexins brassinin, 1-methoxyspirobrassinol methyl eher and cyclobrassinin. Bioorg. Med. Chem. 2013, 21, 6623–6633. [Google Scholar] [CrossRef]
- Mezencev, R.; Kutschy, P.; Salayova, A.; Updegrove, T.; McDonald, J.F. The design, synthesis and anticancer activity of new nitrogen mustard derivatives of natural indole phytoalexin 1-methoxybrassinol. Neoplasma 2009, 56, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Chalal, M.; Delmas, D.; Meunier, P.; Latruffe, N.; Vervandier-Fasseur, D. Inhibition of cancer derived cell lines proliferation by synthesized hydroxylated stilbenes and new ferrocenyl-stilbene analogs. Comparison with resveratrol. Molecules 2014, 19, 7850–7868. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.G.; Rogina, B.; Lavu, S.; Howitz, K.; Helfand, S.L.; Tatar, M.; Sinclair, D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 2004, 430, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Barger, J.L.; Kayo, T.; Vann, J.M.; Arias, E.B.; Wang, J.; Hacker, T.A.; Wang, Y.; Raederstorff, D.; Morrow, J.D.; Leeuwenburgh, C.; et al. A Low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS One 2008, 3, e2264. [Google Scholar] [CrossRef] [PubMed]
- McCalley, A.E.; Kaja, S.; Payne, A.J.; Koulen, P. resveratrol and calcium signaling: Molecular mechanisms and clinical relevance. Molecules 2014, 19, 7327–7340. [Google Scholar] [CrossRef] [PubMed]
- Sanzani, S.M.; Schena, L.; Nigro, F.; de Girolamo, A.; Ippolito, A. Effect of quercetin and umbelliferone on the transcript level of Penicillium expansum genes involved in patulin biosynthesis. Eur. J. Plant Pathol. 2009, 125, 223–233. [Google Scholar] [CrossRef]
- Matthews, D.; Jones, H.; Gans, P.; Coates, S.; Smith, L.M.J. Toxic secondary metabolite production in genetically modified potatoes in response to stress. J. Agric. Food Chem. 2005, 53, 7766–7776. [Google Scholar] [CrossRef] [PubMed]
- Becker, L.; Carré, V.; Poutaraud, A.; Merdinoglu, D.; Chaimbault, P. MALDI mass spectrometry imaging for the simultaneous location of resveratrol, pterostilbene and viniferins on grapevine leaves. Molecules 2014, 19, 10587–10600. [Google Scholar] [CrossRef] [PubMed]
- Marti, G.; Schnee, S.; Andrey, Y.; Simoes-Pires, C.; Carrupt, P.A.; Wolfender, J.L.; Gindro, K. Study of leaf metabolome modifications induced by UV-C radiations in representative Vitis, Cissus and Cannabis species by LC-MS based metabolomics and antioxidant assays. Molecules 2014, 19, 14004–14021. [Google Scholar] [CrossRef] [PubMed]
- Donnez, D.; Jeandet, P.; Clément, C.; Courot, E. Bioproduction of resveratrol and stilbene derivatives by plant cells and microorganisms. Trends Biotechnol. 2009, 27, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Jeandet, P.; Vasserot, Y.; Chastang, T.; Courot, E. Engineering microbial cells for the biosynthesis of natural compounds of pharmaceutical significance. BioMed Res. Int. 2013. [CrossRef]
- Jeandet, P.; Clément, C.; Courot, E. Resveratrol production at large scale using plant cell suspensions. Eng. Life Sci. 2014. [Google Scholar] [CrossRef]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeandet, P.; Hébrard, C.; Deville, M.-A.; Cordelier, S.; Dorey, S.; Aziz, A.; Crouzet, J. Deciphering the Role of Phytoalexins in Plant-Microorganism Interactions and Human Health. Molecules 2014, 19, 18033-18056. https://doi.org/10.3390/molecules191118033
Jeandet P, Hébrard C, Deville M-A, Cordelier S, Dorey S, Aziz A, Crouzet J. Deciphering the Role of Phytoalexins in Plant-Microorganism Interactions and Human Health. Molecules. 2014; 19(11):18033-18056. https://doi.org/10.3390/molecules191118033
Chicago/Turabian StyleJeandet, Philippe, Claire Hébrard, Marie-Alice Deville, Sylvain Cordelier, Stéphan Dorey, Aziz Aziz, and Jérôme Crouzet. 2014. "Deciphering the Role of Phytoalexins in Plant-Microorganism Interactions and Human Health" Molecules 19, no. 11: 18033-18056. https://doi.org/10.3390/molecules191118033
APA StyleJeandet, P., Hébrard, C., Deville, M. -A., Cordelier, S., Dorey, S., Aziz, A., & Crouzet, J. (2014). Deciphering the Role of Phytoalexins in Plant-Microorganism Interactions and Human Health. Molecules, 19(11), 18033-18056. https://doi.org/10.3390/molecules191118033