Structure and Antioxidant Activity Relationships of Isoflavonoids from Dalbergia parviflora
Abstract
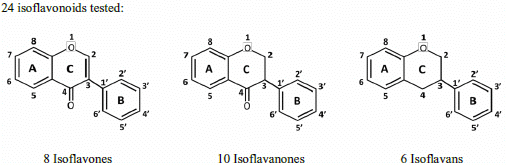
:1. Introduction
2. Results and Discussion
2.1. SAR of D. parviflora Isoflavonoids Based on Xanthine/Xanthine Oxidase Assay
 | No. | Isoflavones | R7 | R5 | R2′ | R3′ | R4′ | R5′ | SC50 (µM) |
| 5 | Calycosin | OH | H | H | OH | OMe | H | 0.25 ± 0.05 | |
| 4 | Khrinone B | OH | OH | OH | H | OMe | OH | 0.60 ± 0.1 | |
| 8 | Khrinone C | OH | OH | OMe | OH | OMe | H | 0.64 ± 0.03 | |
| 3 | Genistein | OH | OH | H | H | OH | H | 9.0 ± 2.2 | |
| 6 | 3′-O-Methylorobol | OH | OH | H | OMe | OH | H | 36.7 ± 7.2 | |
| 7 | Cajanin | OMe | OH | OH | H | OH | H | 54.3 ± 10.7 | |
| 1 | Formononetin | OH | H | H | H | OMe | H | 116.92 ± 15.6 | |
| 2 | Biochanin A | OH | OH | H | H | OMe | H | 203.3 ± 57.6 | |
 | No. | Isoflavanones | R7 | R5 | R2′ | R3′ | R4′ | R5′ | SC50 (µM) |
| 15 | 3(R,S)-Kenusanone G | OH | OH | H | OH | OMe | H | 8.6 ± 1.2 | |
| 12 | 3(R)-7,3′-Dihydroxy-4′-methoxyisoflavanone | OH | H | H | OH | OMe | H | 27.9 ± 5.4 | |
| 14 | Dalparvin B | OH | H | OH | OMe | OMe | H | 30.5 ± 3.8 | |
| 16 | 3(R,S)-Violanone | OH | H | OMe | OH | OMe | H | 43.7 ± 9.7 | |
| 13 | 3(R,S)-Dalparvin | OH | H | OMe | H | OMe | OH | 48.2 ± 15.0 | |
| 11 | 3(R,S)-Onogenin | OH | H | OMe | H | OCH2O | 56.9 ± 0.18 | ||
| 10 | 3(S)-Sativanone | OH | H | OMe | H | OMe | H | 59.3 ± 21.7 | |
| 18 | 3(R)-Dalparvin A | OH | OH | OMe | H | OH | OH | 160.3 ± 54.4 | |
| 17 | 3(S)-Secundiflorol H | OH | OH | OMe | OH | OMe | H | 247.2 ± 82.2 | |
| 9 | 3(R,S)-3′-O-Methyl-violanone | OH | H | OMe | OMe | OMe | H | - | |
 | No. | Isoflavans | R7 | R8 | R2′ | R3′ | R4′ | R5′ | SC50 (µM) |
| 24 | 3(R,S)-3′-Hydroxy-8-methoxy vestitol | OH | OMe | OH | OH | OMe | H | 2.8 ± 0.7 | |
| 21 | (3R)-Vestitol | OH | H | OH | H | OMe | H | 6.4 ± 0.1 | |
| 23 | (3R)(+)-Mucronulatol | OH | H | OMe | OH | OMe | H | 10.0 ± 3.6 | |
| 22 | (3S)-8-Demethylduartin | OH | OH | OMe | OH | OMe | H | 13.4 ± 3.6 | |
| 20 | 3(R,S)-Duartin | OH | OMe | OMe | OH | OMe | H | 12.2 ± 4.2 | |
| 19 | 3(R,S)-Sativan | OH | H | OMe | H | OMe | H | 12.8 ± 1.2 | |
| Chemical Structures | X/XO assay, SC50 (µM) | ORAC assay, Trolox Equivalents (µM TE/10 µM isoflavonoid) | DPPH assay, SC50 (µM) |
|---|---|---|---|
 | 0.64 ± 0.03 | 43.5 ± 3.2 | 61.7 ± 4.5 |
 | 247.2 ± 82.2 | 27.4 ± 7.7 | 74.3 ± 4.2 |
 | 13.4 ± 3.6 | 27.0 ± 1.9 | 115.4 ± 4.3 |
2.2. SAR of D. parviflora Isoflavonoids Based on the ORAC Assay
 | No. | Isoflavones | R7 | R5 | R2′ | R3′ | R4′ | R5′ | (µM TE) † |
| 8 | Khrinone C | OH | OH | OMe | OH | OMe | H | 43.5 ± 3.2 | |
| 5 | Calycosin | OH | H | H | OH | OMe | H | 37.8 ± 1.2 | |
| 3 | Genistein | OH | OH | H | H | OH | H | 37.8 ± 4.5 | |
| 6 | 3′-O-Methylorobol | OH | OH | H | OMe | OH | H | 35.7 ± 5.5 | |
| 7 | Cajanin | OMe | OH | OH | H | OH | H | 34.7 ± 2.2 | |
| 4 | Khrinone B | OH | OH | OH | H | OMe | OH | 34.2 ± 2.9 | |
| 2 | Biochanin A | OH | OH | H | H | OMe | H | 26.6 ± 1.3 | |
| 1 | Formononetin | OH | H | H | H | OMe | H | 2.8 ± 0.5 | |
 | No. | Isoflavones | R7 | R5 | R2′ | R3′ | R4′ | R5′ | (µM TE) † |
| 18 | 3(R)-Dalparvin A | OH | OH | OMe | H | OH | OH | 120.3 ± 15.1 | |
| 15 | 3(R,S)-Kenusanone G | OH | OH | H | OH | OMe | H | 42.1 ± 0.5 | |
| 14 | Dalparvin B | OH | H | OH | OMe | OMe | H | 33.4 ± 4.9 | |
| 16 | 3(R,S)-Violanone | OH | H | OMe | OH | OMe | H | 31.1 ± 2.5 | |
| 12 | 3(R)-7,3′-Dihydroxy-4′-methoxyisoflavanone | OH | H | H | OH | OMe | H | 28.4 ± 7.5 | |
| 17 | 3(S)-Secundiflorol H | OH | OH | OMe | OH | OMe | H | 27.4 ± 7.7 | |
| 13 | 3(R,S)-Dalparvin | OH | H | OMe | H | OMe | OH | 21.8 ± 1.5 | |
| 11 | 3(R,S)-Onogenin | OH | H | OMe | H | OCH2O | - | ||
| 10 | 3(S)-Sativanone | OH | H | OMe | H | OMe | H | - | |
| 9 | 3(R,S)-3′-O-Methyl-violanone | OH | H | OMe | OMe | OMe | H | - | |
 | No. | Isoflavones | R7 | R5 | R2′ | R3′ | R4′ | R5′ | (µM TE) † |
| 21 | 3(R)-Vestitol | OH | H | OH | H | OMe | H | 40.1 ± 1.0 | |
| 23 | 3(R)(+)-Mucronulatol | OH | H | OMe | OH | OMe | H | 39.8 ± 0.5 | |
| 20 | 3(R,S)-Duartin | OH | OMe | OMe | OH | OMe | H | 34.2 ± 0.7 | |
| 24 | 3(R,S)-3′-Hydroxy-8-methoxyvestitol | OH | OMe | OH | OH | OMe | H | 31.4 ± 2.7 | |
| 22 | 3(S)-8-Demethyl-duartin | OH | OH | OMe | OH | OMe | H | 27.0 ± 1.9 | |
| 19 | 3(R,S)-Sativan | OH | H | OMe | H | OMe | H | 24.8 ± 3.1 |

2.3. SAR of D. parviflora Isoflavonoids Based on DPPH Radical Scavenging Activity
 | No. | Isoflavones | R7 | R5 | R2′ | R3′ | R4′ | R5′ | SC50 (µM) |
| 8 | Khrinone C | OH | OH | OMe | OH | OMe | H | 61.7 ± 4.5 | |
| 7 | Cajanin | OMe | OH | OH | H | OH | H | 70.8 ± 1.1 | |
| 6 | 3′-O-Methylorobol | OH | OH | H | OMe | OH | H | 81.2 ± 14.1 | |
| 5 | Calycosin | OH | H | H | OH | OMe | H | 96.2 ± 2.8 | |
| 4 | Khrinone B | OH | OH | OH | H | OMe | OH | 133.6 ± 7.0 | |
| 3 | Genistein | OH | OH | H | H | OH | H | - | |
| 2 | Biochanin A | OH | OH | H | H | OMe | H | - | |
| 1 | Formononetin | OH | H | H | H | OMe | H | - | |
 | No. | Isoflavones | R7 | R5 | R2′ | R3′ | R4′ | R5′ | SC50 (µM) |
| 18 | 3(R)-Dalparvin A | OH | OH | OMe | H | OH | OH | 41.9 ± 4.8 | |
| 17 | 3(3)-Secundiflorol H | OH | OH | OMe | OH | OMe | H | 74.3 ± 4.2 | |
| 12 | 3(R)-7,3′-Dihydroxy-4′-methoxyisoflavanone | OH | H | H | OH | OMe | H | 78.9 ± 1.1 | |
| 13 | 3(R,S)-Dalparvin | OH | H | OMe | H | OMe | OH | 80.4 ± 1.3 | |
| 16 | 3(R,S)-Violanone | OH | H | OMe | OH | OMe | H | 89.7 ± 1.7 | |
| 15 | 3(R,S)-Kenusanone G | OH | OH | H | OH | OMe | H | 111.9 ± 4.7 | |
| 14 | Dalparvin B | OH | H | OH | OMe | OMe | H | 236.3± 9.8 | |
| 11 | 3(RS)-Onogenin | OH | H | OMe | H | OCH2O | - | ||
| 10 | 3(S)-Sativanone | OH | H | OMe | H | OMe | H | - | |
| 9 | 3(R,S)-3′-O-Methyl-violanone | OH | H | OMe | OMe | OMe | H | - | |
 | No. | Isoflavones | R7 | R5 | R2′ | R3′ | R4′ | R5′ | SC50 (µM) |
| 24 | 3(RS)-3′-Hydroxy-8-methoxyvestitol | OH | OMe | OH | OH | OMe | H | 38.7 ± 3.0 | |
| 23 | 3(R)(+)-Mucronulatol | OH | H | OMe | OH | OMe | H | 75.41 ± 3.2 | |
| 22 | 3(3)-8-Demethyl-duartin | OH | OH | OMe | OH | OMe | H | 115.4 ± 4.3 | |
| 21 | 3(3)-Vestitol | OH | H | OH | H | OMe | H | 204.1 ± 8.0 | |
| 19 | 3(R,S)-Sativan | OH | H | OMe | H | OMe | H | - | |
| 20 | 3(R,S)-Duartin | OH | OMe | OMe | OH | OMe | H | - | |
3. Experimental
3.1. Chemicals
3.2. Scavenging of Diphenyl-Picrylhydrazyl (DPPH) Radicals
3.3. Inhibition of Superoxide Radical Formation by Xanthine/Xanthine Oxidase (X/XO Assay)
3.4. Measurement of Oxygen Radical Absorbance Capacity (ORAC)
4. Conclusions
Abbreviations
| SC50 | (50% radical scavenging concentration) |
| ORAC | oxygen radical absorbance capacity |
| AUC | areas under the fluorescence decay curves |
| AAPH | 2,2′-azobis(2-amidinopropane) dihydrochloride |
Acknowledgments
Conflicts of Interest
References
- Williams, R.J.; Spencer, J.P.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Guo, Q.; Rimbach, G.; Moini, H.; Weber, S.; Packer, L. ESR and cell culture studies on free radical-scavenging and antioxidant activities of isoflavonoids. Toxicology 2002, 179, 171–180. [Google Scholar] [CrossRef]
- Choi, J.S.; Chung, H.Y.; Kang, S.S.; Jung, M.J.; Kim, J.W.; No, J.K.; Jung, H.A. The structure-activity relationship of flavonoids as scavengers of peroxynitrite. Phytother. Res. 2002, 16, 232–235. [Google Scholar] [CrossRef]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; van Poel, B.; Pieters, L.; Vlietinck, A.J.; Vanden Berghe, D. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef]
- Yang, J.G.; Liu, B.G.; Liang, G.Z.; Ning, Z.X. Structure-activity relationship of flavonoids active against lard oil oxidation based on quantum chemical analysis. Molecules 2009, 14, 46–52. [Google Scholar]
- Farkas, O.; Jakus, J.; Heberger, K. Quantitative structure-antioxidant activity relationships of flavonoid compounds. Molecules 2004, 9, 1079–1088. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Mei, S.; Jie, X.; Luo, Q.; Corke, H. Structure-radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef]
- Umehara, K.; Nemoto, K.; Matsushita, A.; Terada, E.; Monthakantirat, O.; De-Eknamkul, W.; Miyase, T.; Warashina, T.; Degawa, M.; Noguchi, H. Flavonoids from the heartwood of the Thai medicinal plant Dalbergia parviflora and their effects on estrogenic-responsive human breast cancer cells. J. Nat. Prod. 2009, 72, 2163–2168. [Google Scholar] [CrossRef]
- Umehara, K.; Nemoto, K.; Kimijima, K.; Matsushita, A.; Terada, E.; Monthakantirat, O.; De-Eknamkul, W.; Miyase, T.; Warashina, T.; Degawa, M.; Noguchi, H. Estrogenic constituents of the heartwood of Dalbergia parviflora. Phytochemistry 2008, 69, 546–552. [Google Scholar] [CrossRef]
- McCord, J.M.; Fridovich, I. The reduction of cytochrome c by milk xanthine oxidase. J. Biol. Chem. 1968, 243, 5753–5760. [Google Scholar]
- Sumbayev, V.V. Genistein effect on xanthine oxidase activity. Ukr. Biokhim. Zh. 2001, 73, 39–43. [Google Scholar]
- Nagao, A.; Seki, M.; Kobayashi, H. Inhibition of xanthine oxidase by flavonoids. Biosci. Biotechnol. Biochem. 1999, 63, 1787–1790. [Google Scholar] [CrossRef]
- Yu, D.H.; Bao, Y.M.; An, L.J.; Yang, M. Protection of PC12 cells against superoxide-induced damage by isoflavonoids from Astragalus mongholicus. Biomed. Environ. Sci. 2009, 22, 50–54. [Google Scholar] [CrossRef]
- Wei, H.; Bowen, R.; Cai, Q.; Barnes, S.; Wang, Y. Antioxidant and antipromotional effects of the soybean isoflavone genistein. Proc. Soc. Exp. Biol. Med. 1995, 208, 124–130. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14, 303–311. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Chan, P.T.; Ho, K.Y.; Fung, K.P.; Wang, J. Antioxidant activity of natural flavonoids is governed by number and location of their aromatic hydroxyl groups. Chem. Phys. Lipids 1996, 79, 157–163. [Google Scholar] [CrossRef]
- Yokozawa, T.; Chen, C.P.; Dong, E.; Tanaka, T.; Nonaka, G.I.; Nishioka, I. Study on the inhibitory effect of tannins and flavonoids against the 1,1-diphenyl-2 picrylhydrazyl radical. Biochem. Pharmacol. 1998, 56, 213–222. [Google Scholar] [CrossRef]
- Lien, E.J.; Ren, S.; Bui, H.H.; Wang, R. Quantitative structure-activity relationship analysis of phenolic antioxidants. Free Radic. Biol. Med. 1999, 26, 285–294. [Google Scholar] [CrossRef]
- Sekher Pannala, A.; Chan, T.S.; O’Brien, P.J.; Rice-Evans, C.A. Flavonoid B-ring chemistry and antioxidant activity: Fast reaction kinetics. Biochem. Biophys. Res. Commun. 2001, 282, 1161–1168. [Google Scholar] [CrossRef]
- Silva, M.M.; Santos, M.R.; Caroco, G.; Rocha, R.; Justino, G.; Mira, L. Structure-antioxidant activity relationships of flavonoids: A re-examination. Free Radic. Res. 2002, 36, 1219–1227. [Google Scholar] [CrossRef]
- Johnson, M.K.; Loo, G. Effects of epigallocatechin gallate and quercetin on oxidative damage to cellular DNA. Mutat. Res. 2000, 459, 211–218. [Google Scholar] [CrossRef]
- Matsuda, H.; Wang, T.; Managi, H.; Yoshikawa, M. Structural requirements of flavonoids for inhibition of protein glycation and radical scavenging activities. Bioorg. Med. Chem. 2003, 11, 5317–5323. [Google Scholar] [CrossRef]
- Han, T.; Cheng, G.; Liu, Y.; Yang, H.; Hu, Y.T.; Huang, W. In vitro evaluation of tectoridin, tectorigenin and tectorigenin sodium sulfonate on antioxidant properties. Food Chem. Toxicol. 2012, 50, 409–414. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORAC(FL))) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 51, 3273–3279. [Google Scholar] [CrossRef]
- Sample Availability: All samples are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Promden, W.; Monthakantirat, O.; Umehara, K.; Noguchi, H.; De-Eknamkul, W. Structure and Antioxidant Activity Relationships of Isoflavonoids from Dalbergia parviflora. Molecules 2014, 19, 2226-2237. https://doi.org/10.3390/molecules19022226
Promden W, Monthakantirat O, Umehara K, Noguchi H, De-Eknamkul W. Structure and Antioxidant Activity Relationships of Isoflavonoids from Dalbergia parviflora. Molecules. 2014; 19(2):2226-2237. https://doi.org/10.3390/molecules19022226
Chicago/Turabian StylePromden, Worrawat, Orawan Monthakantirat, Kaoru Umehara, Hiroshi Noguchi, and Wanchai De-Eknamkul. 2014. "Structure and Antioxidant Activity Relationships of Isoflavonoids from Dalbergia parviflora" Molecules 19, no. 2: 2226-2237. https://doi.org/10.3390/molecules19022226
APA StylePromden, W., Monthakantirat, O., Umehara, K., Noguchi, H., & De-Eknamkul, W. (2014). Structure and Antioxidant Activity Relationships of Isoflavonoids from Dalbergia parviflora. Molecules, 19(2), 2226-2237. https://doi.org/10.3390/molecules19022226