Structure-Activity Association of Flavonoids in Lung Diseases
Abstract
:1. Introduction
2. Chemistry and Occurrence of Flavonoids


3. General Aspects and Structure-Activity of Flavonoids in the Oxidative Stress
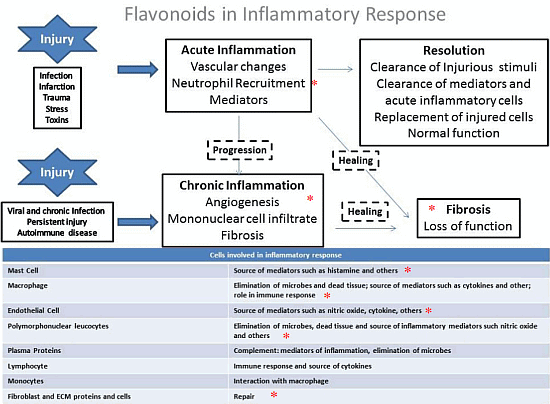
4. General Aspects and the Role of Flavonoids in the Inflammation


5. The Role of Flavonoids in Lung Diseases
5.1. General Aspects
5.2. Chronic Obstructive Pulmonary Disease (COPD)
5.3. Acute Respiratory Distress Syndrome (ARDS)
5.4. Asthma
| Flavonoid | Lung Effect | Reference |
|---|---|---|
| Naringin (1) | Anti-ARDS | [104] |
| Kaempferol (12) | Anti-allergic | [35,109,110] |
| Anti-ARDS | [100] | |
| Anti-asthmathic | [101] | |
| Apigenin (15) | Anti-allergic | [35,109,110] |
| Anti-asthmathic | [71,120] | |
| Genistein (42) | Bronchoconstrictor | [122] |
| Daidzin (43) | Bronchoconstrictor | [123] |
| Daidzein (44) | Bronchoconstrictor | [123] |
| Myricetin (17) | Anti-allergic | [35,109,110] |
| Quercetin (18) | Preventing pulmonar emphysema | [84] |
| Anti-allergic | [35,109,110] | |
| Anti-ARDS | [91] | |
| Anti-asthmathic | [89,114,117] | |
| Luteolin (19) | Anti-allergic | [35,109,110] |
| Anti-asthmathic | [71,114,120] | |
| Anti-COPD | [97,99] | |
| Morin (22) | Anti-asthmathic | [113] |
| Anti-COPD | [85] | |
| Fisetin (24) | Anti-allergic | [35,109,110] |
| Anti-asthmathic | [113] | |
| Anti-COPD | [85] | |
| Scutellarein (25) | Anti-asthmathic | [115] |
| 3,6-Dihydroxyflavone (28) | Anti-allergic | [35,109,110] |
| Flavone (29) | Anti-COPD | [85] |
| Tricetin (34) | Anti-COPD | [85] |
| Quercetagetin (35) | Anti-asthmathic | [56] |
| Kaempferol-3-O-galactoside (36) | Anti-asthmathic | [56] |
| Cirsiliol (37) | Anti-asthmathic | [115] |
| Baicalein (30) | Anti-asthmathic | [115] |
| Isoquercitrin (38) | Anti-asthmathic | [71,117] |
| Liquiritigenin (8) | Anti-COPD | [121] |
| Isoliquiritigenin (45) | Anti-COPD | [121] |
| 7,4’-Dihydroxyflavone (31) | Anti-COPD | [121] |
| Liquiritin apioside (9) | Anti-COPD | [95] |
| Pinocembrin (10) | Anti-ARDS | [102] |
| Oroxylin-A (32) | Act decreasing lung inflamattion | [98] |
| Baicalin (33) | Anti-COPD | [126] |
| Sakuranetin (11) | Anti-asthmathic and COPD | [58,125] |
6. Clinical Perspectives of Flavonoid Application in Lung Disease
7. Conclusions
Acknowledgments
Author Contributions
Conflictts of Interest
References
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Caltagirone, S.; Ranelletti, F.O.; Rinelli, A.; Maggiano, N.; Colasante, A.; Musiani, P.; Aiello, F.B.; Piantelli, M. Interaction with type II estrogen binding sites and antiproliferative activity of tamoxifen and quercetin in human non-small-cell lung cancer. Am. J. Respir. Cell Mol. Biol. 1997, 17, 51–59. [Google Scholar] [CrossRef]
- Ranelletti, F.O.; Ricci, R.; Larocca, L.M.; Maggiano, N.; Capelli, A.; Scambia, G.; Benedetti-Panici, P.; Mancuso, S.; Rumi, C.; Piantelli, M. Growth-inhibitory effect of quercetin and presence of type-II estrogen-binding sites in human colon-cancer cell lines and primary colorectal tumors. Int. J. Cancer 1992, 50, 486–492. [Google Scholar] [CrossRef]
- Yoshida, M.; Sakai, T.; Hosokawa, N.; Marui, N.; Matsumoto, K.; Fujioka, A.; Nishino, H.; Aoike, A. The effect of quercetin on cell cycle progression and growth of human gastric cancer cells. FEBS Lett. 1990, 260, 10–13. [Google Scholar] [CrossRef]
- Lee, K.H.; Yoo, C.G. Simultaneous inactivation of GSK-3beta suppresses quercetin-induced apoptosis by inhibiting the JNK pathway. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 304, L782–L789. [Google Scholar] [CrossRef]
- Croft, K.D. The chemistry and biological effects of flavonoids and phenolic acids. Ann. N. Y. Acad. Sci. 1998, 854, 435–442. [Google Scholar] [CrossRef]
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, flavones, flavanones, and human health: Epidemiological evidence. J. Med. Food 2005, 8, 281–290. [Google Scholar] [CrossRef]
- Veitch, N.C.; Grayer, R.J. Flavonoids and their glycosides, including anthocyanins. Nat. Prod. Rep. 2008, 25, 555–611. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Middleton, E., Jr.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Williams, C.A.; Grayer, R.J. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2004, 21, 539–573. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; van Nood, E.; van Hoorn, D.E.; Boelens, P.G.; van Norren, K.; van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar]
- Beecher, G.R. Overview of dietary flavonoids: Nomenclature, occurrence and intake. J. Nutr. 2003, 133, 3248S–3254S. [Google Scholar]
- Das, D.K. Naturally occurring flavonoids: Structure, chemistry, and high-performance liquid chromatography methods for separation and characterization. Methods Enzymol. 1994, 234, 410–420. [Google Scholar] [CrossRef]
- Jorgensen, R. The origin of land plants: A union of alga and fungus advanced by flavonoids? Biosystems 1993, 31, 193–207. [Google Scholar] [CrossRef]
- Calixto, J.O.B. Biodiversidade como fonte de medicamentos. Ciênc. Cult. 2003, 55, 37–39. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Lien, E.J.; Ren, S.; Bui, H.H.; Wang, R. Quantitative structure-activity relationship analysis of phenolic antioxidants. Free Radic. Biol. Med. 1999, 26, 285–294. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Saran, M. Flavonoids as antioxidants: Determination of radical-scavenging efficiencies. Methods Enzymol. 1990, 186, 343–355. [Google Scholar] [CrossRef]
- Silva, M.M.; Santos, M.R.; Caroco, G.; Rocha, R.; Justino, G.; Mira, L. Structure-antioxidant activity relationships of flavonoids: A re-examination. Free Radic. Res. 2002, 36, 1219–1227. [Google Scholar] [CrossRef]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant and prooxidant behavior of flavonoids: Structure-activity relationships. Free Radic. Biol. Med. 1997, 22, 749–760. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Structure-activity relationships of flavonoids in the cellular antioxidant activity assay. J. Agric. Food Chem. 2008, 56, 8404–8411. [Google Scholar] [CrossRef]
- Noroozi, M.; Angerson, W.J.; Lean, M.E. Effects of flavonoids and vitamin C on oxidative DNA damage to human lymphocytes. Am. J. Clin. Nutr. 1998, 67, 1210–1218. [Google Scholar]
- Ioku, K.; Tsushida, T.; Takei, Y.; Nakatani, N.; Terao, J. Antioxidative activity of quercetin and quercetin monoglucosides in solution and phospholipid bilayers. Biochim. Biophys. Acta 1995, 1234, 99–104. [Google Scholar] [CrossRef]
- Mao, T.K.; Powell, J.; van de Water, J.; Keen, C.L.; Schmitz, H.H.; Hammerstone, J.F.; Gershwin, M.E. The effect of cocoa procyanidins on the transcription and secretion of interleukin 1 beta in peripheral blood mononuclear cells. Life Sci. 2000, 66, 1377–1386. [Google Scholar] [CrossRef]
- Burda, S.; Oleszek, W. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef]
- Huk, I.; Brovkovych, V.; Nanobash Vili, J.; Weigel, G.; Neumayer, C.; Partyka, L.; Patton, S.; Malinski, T. Bioflavonoid quercetin scavenges superoxide and increases nitric oxide concentration in ischaemia-reperfusion injury: An experimental study. Br. J. Surg. 1998, 85, 1080–1085. [Google Scholar] [CrossRef]
- Shutenko, Z.; Henry, Y.; Pinard, E.; Seylaz, J.; Potier, P.; Berthet, F.; Girard, P.; Sercombe, R. Influence of the antioxidant quercetin in vivo on the level of nitric oxide determined by electron paramagnetic resonance in rat brain during global ischemia and reperfusion. Biochem. Pharmacol. 1999, 57, 199–208. [Google Scholar] [CrossRef]
- Van Acker, S.A.; Tromp, M.N.; Haenen, G.R.; van der Vijgh, W.J.; Bast, A. Flavonoids as scavengers of nitric oxide radical. Biochem. Biophys. Res. Commun. 1995, 214, 755–759. [Google Scholar] [CrossRef]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; van Poel, B.; Pieters, L.; Vlietinck, A.J.; Vanden Berghe, D. Structure-activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef]
- Ferrali, M.; Signorini, C.; Caciotti, B.; Sugherini, L.; Ciccoli, L.; Giachetti, D.; Comporti, M. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity. FEBS Lett. 1997, 416, 123–129. [Google Scholar] [CrossRef]
- Friesenecker, B.; Tsai, A.G.; Intaglietta, M. Cellular basis of inflammation, edema and the activity of Daflon 500 mg. Int. J. Microcirc. Clin. Exp. 1995, 15 (Suppl. 1), 1167–1179. [Google Scholar]
- Middleton, E., Jr.; Kandaswami, C. Effects of flavonoids on immune and inflammatory cell functions. Biochem. Pharmacol. 1992, 43, 1167–1179. [Google Scholar] [CrossRef]
- Zhu, B.T.; Ezell, E.L.; Liehr, J.G. Catechol-O-methyltransferase-catalyzed rapid O-methylation of mutagenic flavonoids. Metabolic inactivation as a possible reason for their lack of carcinogenicity in vivo. J. Biol. Chem. 1994, 269, 292–299. [Google Scholar]
- Fiander, H.; Schneider, H. Dietary ortho phenols that induce glutathione S-transferase and increase the resistance of cells to hydrogen peroxide are potential cancer chemopreventives that act by two mechanisms: The alleviation of oxidative stress and the detoxification of mutagenic xenobiotics. Cancer Lett. 2000, 156, 117–124. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.; Sterk, P.J.; Gaston, B.; Folkerts, G. Nitric oxide in health and disease of the respiratory system. Physiol. Rev. 2004, 84, 731–65. [Google Scholar] [CrossRef]
- Matot, I.; Sprung, C.L. Definition of sepsis. Intensive Care Med. 2001, 27 (Suppl. 1), S3–S9. [Google Scholar] [CrossRef]
- Wheeler, A.P.; Bernard, G.R. Treating patients with severe sepsis. N Engl. J. Med. 1999, 340, 207–214. [Google Scholar] [CrossRef]
- Pajkrt, D.; van der Poll, T.; Levi, M.; Cutler, D.L.; Affrime, M.B.; van den Ende, A.; ten Cate, J.W.; van Deventer, S.J. Interleukin-10 inhibits activation of coagulation and fibrinolysis during human endotoxemia. Blood 1997, 89, 2701–2705. [Google Scholar]
- Hesslinger, C.; Strub, A.; Boer, R.; Ulrich, W.R.; Lehner, M.D.; Braun, C. Inhibition of inducible nitric oxide synthase in respiratory diseases. Biochem. Soc. Trans. 2009, 37, 886–891. [Google Scholar] [CrossRef]
- Holgate, S.T. Asthma: A dynamic disease of inflammation and repair. Ciba Found. Symp. 1997, 206, 5–28, discussion 28–34, 106–110. [Google Scholar]
- Hantos, Z.; Adamicza, A.; Janosi, T.Z.; Szabari, M.V.; Tolnai, J.; Suki, B. Lung volumes and respiratory mechanics in elastase-induced emphysema in mice. J. Appl. Physiol. 2008, 105, 1864–1872. [Google Scholar] [CrossRef]
- Prado, C.M.; Leick-Maldonado, E.A.; Yano, L.; Leme, A.S.; Capelozzi, V.L.; Martins, M.A.; Tiberio, I.F. Effects of nitric oxide synthases in chronic allergic airway inflammation and remodeling. Am. J. Respir. Cell Mol. Biol. 2006, 35, 457–465. [Google Scholar]
- Prado, C.M.; Leick-Maldonado, E.A.; Kasahara, D.I.; Capelozzi, V.L.; Martins, M.A.; Tiberio, I.F. Effects of acute and chronic nitric oxide inhibition in an experimental model of chronic pulmonary allergic inflammation in guinea pigs. Am. J. Physiol. Lung Cell Mol. Physiol. 2005, 289, L677–L683. [Google Scholar] [CrossRef]
- Ricciardolo, F.L.; di Stefano, A.; Sabatini, F.; Folkerts, G. Reactive nitrogen species in the respiratory tract. Eur. J. Pharmacol. 2006, 533, 240–252. [Google Scholar] [CrossRef]
- Smith, W.L.; DeWitt, D.L.; Garavito, R.M. Cyclooxygenases: Structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000, 69, 145–182. [Google Scholar] [CrossRef]
- Rang, H.P.; Dale, M.M.; Ritter, J.M.; Flower, R.J. Farmacologia, 6th ed.; Elsevier/Medicina Nacionais: Rio de Janeiro, Brasil, 2007. [Google Scholar]
- Lin, S.Y.; Tsai, S.J.; Wang, L.H.; Wu, M.F.; Lee, H. Protection by quercetin against cooking oil fumes-induced DNA damage in human lung adenocarcinoma CL-3 cells: Role of COX-2. Nutr. Cancer 2002, 44, 95–101. [Google Scholar] [CrossRef]
- Bastianetto, S.; Zheng, W.H.; Quirion, R. Neuroprotective abilities of resveratrol and other red wine constituents against nitric oxide-related toxicity in cultured hippocampal neurons. Br. J. Pharmacol. 2000, 131, 711–720. [Google Scholar] [CrossRef]
- Lee, S.C.; Kuan, C.Y.; Yang, C.C.; Yang, S.D. Bioflavonoids commonly and potently induce tyrosine dephosphorylation/inactivation of oncogenic proline-directed protein kinase FA in human prostate carcinoma cells. Anticancer Res. 1998, 18, 1117–1121. [Google Scholar]
- Ferrandiz, M.L.; Alcaraz, M.J. Anti-inflammatory activity and inhibition of arachidonic acid metabolism by flavonoids. Agents Actions 1991, 32, 283–288. [Google Scholar] [CrossRef]
- Ferrandiz, M.L.; Nair, A.G.; Alcaraz, M.J. Inhibition of sheep platelet arachidonate metabolism by flavonoids from Spanish and Indian medicinal herbs. Pharmazie 1990, 45, 206–208. [Google Scholar]
- Laughton, M.J.; Evans, P.J.; Moroney, M.A.; Hoult, J.R.; Halliwell, B. Inhibition of mammalian 5-lipoxygenase and cyclo-oxygenase by flavonoids and phenolic dietary additives. Relationship to antioxidant activity and to iron ion-reducing ability. Biochem. Pharmacol. 1991, 42, 1673–1681. [Google Scholar] [CrossRef]
- Yoshimoto, T.; Furukawa, M.; Yamamoto, S.; Horie, T.; Watanabe-Kohno, S. Flavonoids: Potent inhibitors of arachidonate 5-lipoxygenase. Biochem. Biophys. Res. Commun. 1983, 116, 612–618. [Google Scholar] [CrossRef]
- Santangelo, C.; Vari, R.; Scazzocchio, B.; di Benedetto, R.; Filesi, C.; Masella, R. Polyphenols, intracellular signalling and inflammation. Ann. Ist. Super Sanita 2007, 43, 394–405. [Google Scholar]
- Toledo, A.C.; Sakoda, C.P.; Perini, A.; Pinheiro, N.M.; Magalhaes, R.M.; Grecco, S.; Tiberio, I.F.; Camara, N.O.; Martins, M.A.; Lago, J.H.; et al. Flavonone treatment reverses airway inflammation and remodelling in an asthma murine model. Br. J. Pharmacol. 2013, 168, 1736–1749. [Google Scholar] [CrossRef]
- Jiang, J.; Mo, Z.C.; Yin, K.; Zhao, G.J.; Lv, Y.C.; Ouyang, X.P.; Jiang, Z.S.; Fu, Y.; Tang, C.K. Epigallocatechin-3-gallate prevents TNF-alpha-induced NF-kappaB activation thereby upregulating ABCA1 via the Nrf2/Keap1 pathway in macrophage foam cells. Int. J. Mol. Med. 2012, 29, 946–956. [Google Scholar]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Invest. 2001, 107, 7–11. [Google Scholar] [CrossRef]
- Lopez-Posadas, R.; Ballester, I.; Abadia-Molina, A.C.; Suarez, M.D.; Zarzuelo, A.; Martinez-Augustin, O.; Sanchez de Medina, F. Effect of flavonoids on rat splenocytes, a structure-activity relationship study. Biochem. Pharmacol. 2008, 76, 495–506. [Google Scholar] [CrossRef]
- Kim, H.P.; Son, K.H.; Chang, H.W.; Kang, S.S. Anti-inflammatory plant flavonoids and cellular action mechanisms. J. Pharmacol. Sci. 2004, 96, 229–245. [Google Scholar] [CrossRef]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Cazarolli, L.H.; Zanatta, L.; Alberton, E.H.; Figueiredo, M.S.; Folador, P.; Damazio, R.G.; Pizzolatti, M.G.; Silva, F.R. Flavonoids: Prospective drug candidates. Mini Rev. Med. Chem. 2008, 8, 1429–1440. [Google Scholar]
- Biesalski, H.K. Polyphenols and inflammation: Basic interactions. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 724–728. [Google Scholar] [CrossRef]
- Yoon, J.H.; Baek, S.J. Molecular targets of dietary polyphenols with anti-inflammatory properties. Yonsei. Med. J. 2005, 46, 585–596. [Google Scholar] [CrossRef]
- O’Leary, K.A.; de Pascual-Tereasa, S.; Needs, P.W.; Bao, Y.P.; O’Brien, N.M.; Williamson, G. Effect of flavonoids and vitamin E on cyclooxygenase-2 (COX-2) transcription. Mutat. Res. 2004, 551, 245–254. [Google Scholar] [CrossRef]
- Coutinho, M.A.S.; Muzitano, M.F.; Costa, S.N.S. Flavonóides: Potenciais agentes terapêuticos para o processo inflamatório. Rev. Virtual Quím. 2009, 1, 241–256. [Google Scholar]
- Robak, J.; Gryglewski, R.J. Bioactivity of flavonoids. Pol J. Pharmacol. 1996, 48, 555–564. [Google Scholar]
- Kim, H.P.; Mani, I.; Iversen, L.; Ziboh, V.A. Effects of naturally-occurring flavonoids and biflavonoids on epidermal cyclooxygenase and lipoxygenase from guinea-pigs. Prostaglandins Leukot. Essent. Fatty Acids 1998, 58, 17–24. [Google Scholar] [CrossRef]
- Hirano, T.; Higa, S.; Arimitsu, J.; Naka, T.; Shima, Y.; Ohshima, S.; Fujimoto, M.; Yamadori, T. Kawase, I.; Tanaka, T. Flavonoids such as luteolin, fisetin and apigenin are inhibitors of interleukin-4 and interleukin-13 production by activated human basophils. Int. Arch. Allergy Immunol. 2004, 134, 135–140. [Google Scholar] [CrossRef]
- Lattig, J.; Bohl, M.; Fischer, P.; Tischer, S.; Tietbohl, C.; Menschikowski, M.; Gutzeit, H.O.; Metz, P.; Pisabarro, M.T. Mechanism of inhibition of human secretory phospholipase A2 by flavonoids: Rationale for lead design. J. Comput. Aided Mol. Des. 2007, 21, 473–483. [Google Scholar] [CrossRef]
- Rotelli, A.E.; Guardia, T.; Juarez, A.O.; de la Rocha, N.E.; Pelzer, L.E. Comparative study of flavonoids in experimental models of inflammation. Pharmacol. Res. 2003, 48, 601–606. [Google Scholar] [CrossRef]
- Moroney, M.A.; Alcaraz, M.J.; Forder, R.A.; Carey, F.; Hoult, J.R. Selectivity of neutrophil 5-lipoxygenase and cyclo-oxygenase inhibition by an anti-inflammatory flavonoid glycoside and related aglycone flavonoids. J. Pharm. Pharmacol. 1988, 40, 787–792. [Google Scholar]
- Hollman, P.C.; Katan, M.B. Dietary flavonoids: Intake, health effects and bioavailability. Food Chem. Toxicol. 1999, 37, 937–942. [Google Scholar] [CrossRef]
- Bae, E.A.; Han, M.J.; Lee, M.; Kim, D.H. In vitro inhibitory effect of some flavonoids on rotavirus infectivity. Biol. Pharm. Bull. 2000, 23, 1122–1124. [Google Scholar] [CrossRef]
- Lim, H.J.; Jin, H.G.; Woo, E.R.; Lee, S.K.; Kim, H.P. The root barks of Morus alba and the flavonoid constituents inhibit airway inflammation. J. Ethnopharmacol. 2013, 149, 169–175. [Google Scholar] [CrossRef]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef]
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2014. Available online: http://www.goldcopd.org/ (accessed on 4 March 2014).
- MacNee, W.; Rahman, I. Is oxidative stress central to the pathogenesis of chronic obstructive pulmonary disease? Trends Mol. Med. 2001, 7, 55–62. [Google Scholar] [CrossRef]
- Nowak, D.; Kasielski, M.; Antczak, A.; Pietras, T.; Bialasiewicz, P. Increased content of thiobarbituric acid-reactive substances and hydrogen peroxide in the expired breath condensate of patients with stable chronic obstructive pulmonary disease: No significant effect of cigarette smoking. Respir. Med. 1999, 93, 389–396. [Google Scholar] [CrossRef]
- Lagente, V.; Manoury, B.; Nenan, S.; le Quement, C.; Martin-Chouly, C.; Boichot, E. Role of matrix metalloproteinases in the development of airway inflammation and remodeling. Braz. J. Med. Biol. Res. 2005, 38, 1521–1530. [Google Scholar] [CrossRef]
- Demedts, I.K.; Demoor, T.; Bracke, K.R.; Joos, G.F.; Brusselle, G.G. Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema. Respir. Res. 2006, 7, 53. [Google Scholar] [CrossRef]
- Tabak, C.; Arts, I.C.; Smit, H.A.; Heederik, D.; Kromhout, D. Chronic obstructive pulmonary disease and intake of catechins, flavonols, and flavones: The MORGEN Study. Am. J. Respir. Crit. Care Med. 2001, 164, 61–64. [Google Scholar] [CrossRef]
- Weseler, A.R.; Geraets, L.; Moonen, H.J.J.; Manders, R.J.F.; van Loon, L.J.C.; Pennings, H.-J.; Wouters, E.F.M.; Bast, A.; Hageman, G.J. Poly (ADP-ribose) polymerase-1-inhibiting Flavonoids Attenuate Cytokine Release in Blood from Male Patients with Chronic Obstructive Pulmonary Disease or Type 2 Diabetes. J. Nutr. 2009, 139, 952–957. [Google Scholar] [CrossRef]
- Park, H.K.; Kim, S.J.; Kwon, D.Y.; Park, J.H.; Kim, Y.C. Protective effect of quercetin against paraquat-induced lung injury in rats. Life Sci. 2010, 87, 181–186. [Google Scholar] [CrossRef]
- Chirumbolo, S. The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflamm. Allergy Drug Targets 2010, 9, 263–285. [Google Scholar] [CrossRef]
- Hayashi, Y.; Matsushima, M.; Nakamura, T.; Shibasaki, M.; Hashimoto, N.; Imaizumi, K.; Shimokata, K.; Hasegawa, Y.; Kawabe, T. Quercetin protects against pulmonary oxidant stress via heme oxygenase-1 induction in lung epithelial cells. Biochem. Biophys. Res. Commun. 2012, 417, 169–174. [Google Scholar] [CrossRef]
- Li, N.; Li, Q.; Zhou, X.D.; Kolosov, V.P.; Perelman, J.M. The effect of quercetin on human neutrophil elastase-induced mucin5AC expression in human airway epithelial cells. Int. Immunopharmacol. 2012, 14, 195–201. [Google Scholar] [CrossRef]
- Rakotomalala, G.; Agard, C.; Tonnerre, P.; Tesse, A.; Derbre, S.; Michalet, S.; Hamzaoui, J.; Rio, M.; Cario-Toumaniantz, C.; Richomme, P.; et al. Extract from Mimosa pigra attenuates chronic experimental pulmonary hypertension. J. Ethnopharmacol. 2013, 148, 106–116. [Google Scholar] [CrossRef]
- Johansson, S.; Goransson, U.; Luijendijk, T.; Backlund, A.; Claeson, P.; Bohlin, L. A neutrophil multitarget functional bioassay to detect anti-inflammatory natural products. J. Nat. Prod. 2002, 65, 32–41. [Google Scholar] [CrossRef]
- Kanashiro, A.; Souza, J.G.; Kabeya, L.M.; Azzolini, A.E.; Lucisano-Valim, Y.M. Elastase release by stimulated neutrophils inhibited by flavonoids: Importance of the catechol group. Z Naturforsch. C 2007, 62, 357–361. [Google Scholar]
- Walker, E.H.; Pacold, M.E.; Perisic, O.; Stephens, L.; Hawkins, P.T.; Wymann, M.P.; Williams, R.L. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol. Cell 2000, 6, 909–919. [Google Scholar] [CrossRef]
- Ganesan, S.; Faris, A.N.; Comstock, A.T.; Chattoraj, S.S.; Chattoraj, A.; Burgess, J.R.; Curtis, J.L.; Martinez, F.J.; Zick, S.; Hershenson, M.B.; et al. Quercetin prevents progression of disease in elastase/LPS-exposed mice by negatively regulating MMP expression. Respir. Res. 2010, 11, 131. [Google Scholar] [CrossRef]
- Guan, Y.; Li, F.F.; Hong, L.; Yan, X.F.; Tan, G.L.; He, J.S.; Dong, X.W.; Bao, M.J.; Xie, Q.M. Protective effects of liquiritin apioside on cigarette smoke-induced lung epithelial cell injury. Fundam. Clin. Pharmacol. 2011, 26, 473–483. [Google Scholar]
- Fanelli, V.; Vlachou, A.; Ghannadian, S.; Simonetti, U.; Slutsky, A.S.; Zhang, H. Acute respiratory distress syndrome: New definition, current and future therapeutic options. J. Thorac. Dis. 2013, 5, 326–334. [Google Scholar]
- Chen, J.; Wang, J.B.; Yu, C.H.; Chen, L.Q.; Xu, P.; Yu, W.Y. Total flavonoids of Mosla scabra leaves attenuates lipopolysaccharide-induced acute lung injury via down-regulation of inflammatory signaling in mice. J. Ethnopharmacol. 2013, 148, 835–841. [Google Scholar] [CrossRef]
- Tseng, T.L.; Chen, M.F.; Tsai, M.J.; Hsu, Y.H.; Chen, C.P.; Lee, T.J. Oroxylin-A rescues LPS-induced acute lung injury via regulation of NF-kappaB signaling pathway in rodents. PLoS One 2012, 7, e47403. [Google Scholar]
- Kuo, M.Y.; Liao, M.F.; Chen, F.L.; Li, Y.C.; Yang, M.L.; Lin, R.H.; Kuan, Y.H. Luteolin attenuates the pulmonary inflammatory response involves abilities of antioxidation and inhibition of MAPK and NFkappaB pathways in mice with endotoxin-induced acute lung injury. Food Chem. Toxicol. 2011, 49, 2660–2666. [Google Scholar] [CrossRef]
- Chen, X.; Yang, X.; Liu, T.; Guan, M.; Feng, X.; Dong, W.; Chu, X.; Liu, J.; Tian, X.; Ci, X.; et al. Kaempferol regulates MAPKs and NF-kappaB signaling pathways to attenuate LPS-induced acute lung injury in mice. Int. Immunopharmacol. 2012, 14, 209–216. [Google Scholar] [CrossRef]
- Gong, J.H.; Shin, D.; Han, S.Y.; Park, S.H.; Kang, M.K.; Kim, J.L.; Kang, Y.H. Blockade of airway inflammation by kaempferol via disturbing Tyk-STAT signaling in airway epithelial Cells and in asthmatic mice. Evid. Based Complement. Alternat. Med. 2013, 2013, 250725. [Google Scholar]
- Soromou, L.W.; Chu, X.; Jiang, L.; Wei, M.; Huo, M.; Chen, N.; Guan, S.; Yang, X.; Chen, C.; Feng, H.; et al. In vitro and in vivo protection provided by pinocembrin against lipopolysaccharide-induced inflammatory responses. Int. Immunopharmacol. 2012, 14, 66–74. [Google Scholar] [CrossRef]
- Li, H.B.; Chen, F. Isolation and purification of baicalein, wogonin and oroxylin A from the medicinal plant Scutellaria baicalensis by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1074, 107–110. [Google Scholar]
- Chen, Y.; Nie, Y.C.; Luo, Y.L.; Lin, F.; Zheng, Y.F.; Cheng, G.H.; Wu, H.; Zhang, K.J.; Su, W.W.; Shen, J.G.; et al. Protective effects of naringin against paraquat-induced acute lung injury and pulmonary fibrosis in mice. Food Chem. Toxicol. 2013, 58, 133–140. [Google Scholar] [CrossRef]
- Bateman, E.D.; Hurd, S.S.; Barnes, P.J.; Bousquet, J.; Drazen, J.M.; FitzGerald, M.; Gibson, P.; Ohta, K.; O’Byrne, P.; Pedersen, S.E.; et al. Global strategy for asthma management and prevention: GINA executive summary. Eur. Respir. J. 2008, 31, 143–178. [Google Scholar] [CrossRef]
- Masoli, M.; Fabian, D.; Holt, S.; Beasley, R. The global burden of asthma: Executive summary of the GINA dissemination committee report. Allergy 2004, 59, 469–478. [Google Scholar] [CrossRef]
- Devereux, G.; Seaton, A. Diet as a risk factor for atopy and asthma. J. Allergy Clin. Immunol. 2005, 115, 1109–1117. [Google Scholar] [CrossRef]
- Knekt, P.; Kumpulainen, J.; Jarvinen, R.; Rissanen, H.; Heliovaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar]
- Fewtrell, C.M.; Gomperts, B.D. Effect of flavone inhibitors of transport ATPases on histamine secretion from rat mast cells. Nature 1977, 265, 635–636. [Google Scholar] [CrossRef]
- Middleton, E., Jr.; Drzewiecki, G.; Krishnarao, D. Quercetin: An inhibitor of antigen-induced human basophil histamine release. J. Immunol. 1981, 127, 546–550. [Google Scholar]
- Cheong, H.; Ryu, S.Y.; Oak, M.H.; Cheon, S.H.; Yoo, G.S.; Kim, K.M. Studies of structure activity relationship of flavonoids for the anti-allergic actions. Arch. Pharm. Res. 1998, 21, 478–480. [Google Scholar] [CrossRef]
- Townsend, E.A.; Emala, C.W., Sr. Quercetin acutely relaxes airway smooth muscle and potentiates beta-agonist-induced relaxation via dual phosphodiesterase inhibition of PLCbeta and PDE4. Am. J. Physiol. Lung Cell Mol. Physiol. 2013, 305, L396–L403. [Google Scholar] [CrossRef]
- Jung, C.H.; Lee, J.Y.; Park, J.H.; Cho, B.J.; Sim, S.S.; Kim, C.J. Flavonols attenuate the immediate and late-phase asthmatic responses to aerosolized-ovalbumin exposure in the conscious guinea pig. Fitoterapia 2010, 81, 803–812. [Google Scholar] [CrossRef]
- Kimata, M.; Shichijo, M.; Miura, T.; Serizawa, I.; Inagaki, N.; Nagai, H. Effects of luteolin, quercetin and baicalein on immunoglobulin E-mediated mediator release from human cultured mast cells. Clin. Exp. Allergy 2000, 30, 501–508. [Google Scholar] [CrossRef]
- Kimata, M.; Inagaki, N.; Nagai, H. Effects of luteolin and other flavonoids on IgE-mediated allergic reactions. Planta Med. 2000, 66, 25–29. [Google Scholar] [CrossRef]
- Nanua, S.; Zick, S.M.; Andrade, J.E.; Sajjan, U.S.; Burgess, J.R.; Lukacs, N.W.; Hershenson, M.B. Quercetin blocks airway epithelial cell chemokine expression. Am. J. Respir. Cell Mol. Biol. 2006, 35, 602–610. [Google Scholar] [CrossRef]
- Rogerio, A.P.; Kanashiro, A.; Fontanari, C.; da Silva, E.V.; Lucisano-Valim, Y.M.; Soares, E.G.; Faccioli, L.H. Anti-inflammatory activity of quercetin and isoquercitrin in experimental murine allergic asthma. Inflamm. Res. 2007, 56, 402–408. [Google Scholar] [CrossRef]
- Higa, S.; Hirano, T.; Kotani, M.; Matsumoto, M.; Fujita, A.; Suemura, M.; Kawase, I.; Tanaka, T. Fisetin, a flavonol, inhibits TH2-type cytokine production by activated human basophils. J. Allergy Clin. Immunol. 2003, 111, 1299–1306. [Google Scholar] [CrossRef]
- Hirano, T.; Higa, S.; Arimitsu, J.; Naka, T.; Ogata, A.; Shima, Y.; Fujimoto, M.; Yamadori, T.; Ohkawara, T.; Kuwabara, Y.; et al. uteolin, a flavonoid, inhibits AP-1 activation by basophils. Biochem. Biophys. Res. Commun. 2006, 340, 1–7. [Google Scholar] [CrossRef]
- Mastuda, H.; Morikawa, T.; Ueda, K.; Managi, H.; Yoshikawa, M. Structural requirements of flavonoids for inhibition of antigen-Induced degranulation, TNF-alpha and IL-4 production from RBL-2H3 cells. Bioorg. Med. Chem. 2002, 10, 3123–3128. [Google Scholar] [CrossRef]
- Jayaprakasam, B.; Doddaga, S.; Wang, R.; Holmes, D.; Goldfarb, J.; Li, X.M. Licorice flavonoids inhibit eotaxin-1 secretion by human fetal lung fibroblasts in vitro. J. Agric. Food Chem. 2009, 57, 820–825. [Google Scholar] [CrossRef]
- Duan, W.; Kuo, I.C.; Selvarajan, S.; Chua, K.Y.; Bay, B.H.; Wong, W.S. Antiinflammatory effects of genistein, a tyrosine kinase inhibitor, on a guinea pig model of asthma. Am. J. Respir. Crit. Care Med. 2003, 167, 185–192. [Google Scholar] [CrossRef]
- Bao, Z.S.; Hong, L.; Guan, Y.; Dong, X.W.; Zheng, H.S.; Tan, G.L.; Xie, Q.M. Inhibition of airway inflammation, hyperresponsiveness and remodeling by soy isoflavone in a murine model of allergic asthma. Int. Immunopharmacol. 2011, 11, 899–906. [Google Scholar] [CrossRef]
- Hernandez, V.; Recio, M.C.; Manez, S.; Giner, R.M.; Rios, J.L. Effects of naturally occurring dihydroflavonols from Inula viscosa on inflammation and enzymes involved in the arachidonic acid metabolism. Life Sci. 2007, 81, 480–488. [Google Scholar] [CrossRef]
- Taguchi, L.; Pinheiro, N.; Olivo, C.; Grecco, S.; Choqueta-Toledo, A.; Lopes, F.; Martins, M.; Tiberio, I.; Lago, J.; Prado, C. 5,6,7-Trihydroxy-4-methoxy.-flavanone from Baccharis. retusa. (Asteraceae.) attenuates elastase-induced emphysema in mice. Am. J. Respir. Crit. Care. Med. 2012, 185, A6551. [Google Scholar]
- Li, L.; Bao, H.; Wu, J.; Duan, X.; Liu, B.; Sun, J.; Gong, W.; Lv, Y.; Zhang, H.; Luo, Q.; et al. Baicalin is anti-inflammatory in cigarette smoke-induced inflammatory models in vivo and in vitro: A possible role for HDAC2 activity. Int. Immunopharmacol. 2012, 13, 15–22. [Google Scholar] [CrossRef]
- Kalhan, R.; Smith, L.J.; Nlend, M.C.; Nair, A.; Hixon, J.L.; Sporn, P.H. A mechanism of benefit of soy genistein in asthma: Inhibition of eosinophil p38-dependent leukotriene synthesis. Clin. Exp. Allergy 2008, 38, 103–112. [Google Scholar]
- Smith, L.J.; Holbrook, J.T.; Wise, R.; Blumenthal, M.; Dozor, A.J.; Mastronarde, J.; Williams, L. Dietary intake of soy genistein is associated with lung function in patients with asthma. J. Asthma 2004, 41, 833–843. [Google Scholar] [CrossRef]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lago, J.H.G.; Toledo-Arruda, A.C.; Mernak, M.; Barrosa, K.H.; Martins, M.A.; Tibério, I.F.L.C.; Prado, C.M. Structure-Activity Association of Flavonoids in Lung Diseases. Molecules 2014, 19, 3570-3595. https://doi.org/10.3390/molecules19033570
Lago JHG, Toledo-Arruda AC, Mernak M, Barrosa KH, Martins MA, Tibério IFLC, Prado CM. Structure-Activity Association of Flavonoids in Lung Diseases. Molecules. 2014; 19(3):3570-3595. https://doi.org/10.3390/molecules19033570
Chicago/Turabian StyleLago, João Henrique G., Alessandra C. Toledo-Arruda, Márcia Mernak, Kaidu H. Barrosa, Milton A. Martins, Iolanda F. L. C. Tibério, and Carla M. Prado. 2014. "Structure-Activity Association of Flavonoids in Lung Diseases" Molecules 19, no. 3: 3570-3595. https://doi.org/10.3390/molecules19033570
APA StyleLago, J. H. G., Toledo-Arruda, A. C., Mernak, M., Barrosa, K. H., Martins, M. A., Tibério, I. F. L. C., & Prado, C. M. (2014). Structure-Activity Association of Flavonoids in Lung Diseases. Molecules, 19(3), 3570-3595. https://doi.org/10.3390/molecules19033570