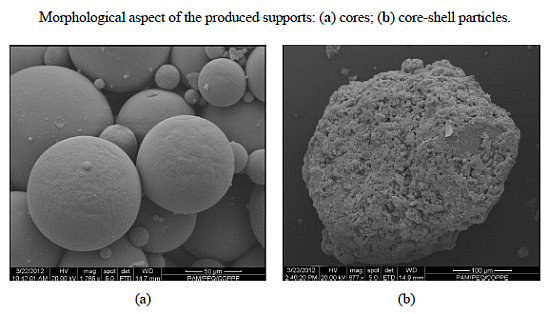
Influence of the Morphology of Core-Shell Supports on the Immobilization of Lipase B from Candida antarctica
Abstract
:1. Introduction
2. Results and Discussion
2.1. CAL-B Immobilization


2.2. Influence of the Morphology of the Supports on the Immobilization Parameters
| Supports | Ue (U/gsupport) | Ahid (U/gbio) | Aester (U/gbio) | Utheo (U/gsupport) | Η (%) | Ra (%) | Protein Concentration on the Support (mg/gsupport) |
|---|---|---|---|---|---|---|---|
| Accurel | 91.8 | 0.7 ± 0.7 | 616 ± 166 | 91.4 | 99.5 | 0.7 | 4.5 |
| Carrier 1 | 127.0 | 2.3 ± 0.6 | 588 ± 307 | 126.0 | 99.2 | 1.8 | 5.2 |
| Carrier 2 | 84.4 | 5.7 ± 2.4 | 133 ± 111 | 32.4 | 38.4 | 17.6 | 0.5 |
| Carrier 3 | 116.8 | 4.2 ± 2.5 | 311 ± 80 | 115.0 | 98.4 | 3.6 | 5.3 |
| Carrier 4 | 80.7 | 2.2 ± 1.1 | 56 ± 21 | 34.8 | 43.1 | 6.3 | 1.0 |
| Carrier 5 | 91.6 | 0.9 ± 0.9 | 193 ± 40 | 85.6 | 93.5 | 1.1 | 3.7 |
| Carrier 6 | - | - | - | - | - | - | - |
| Carrier 7 | 84.2 | 0.9 ± 0.4 | 104 ± 26 | 21.9 | 26.0 | 3.9 | 1.5 |
| Carrier 12 | 73.5 | 7.1 ± 2.4 | 828 ± 397 | 69.3 | 94.2 | 10.3 | 1.5 |
| Carrier 8 | 82.1 | 1.9 ± 0.3 | 315 ± 59 | 77.3 | 94.2 | 2.4 | 4.3 |
| Carrier 9 | 82.1 | 1.1 ± 0.5 | 175 ± 3 | 76.5 | 93.2 | 1.5 | 4.3 |
| Carrier 10 | 83.5 | 2.1 ± 0.3 | 186 ± 43 | 71.7 | 85.9 | 2.9 | 3.9 |
| Carrier 11 | 83.0 | 1.0 ± 0.3 | 165 ± 104 | 80.8 | 97.4 | 1.2 | 4.5 |
| Carrier 13 | 76.0 | 3.5 ± 0.6 | 929 ± 231 | 70.2 | 92.5 | 4.9 | 2.1 |
| Carrier 14 | 80.6 | 1.6 ± 0.5 | 364 ± 156 | 73.3 | 90.9 | 2.2 | 2.4 |
| Carrier 15 | 81.4 | 4.5 ± 1.0 | 234 ± 90 | 17.6 | 21.6 | 25.7 | 0.5 |
| Carrier 16 | 81.6 | 4.4 ± 1.0 | 512 ± 197 | 64.2 | 78.6 | 6.8 | 2.4 |
| Carrier 17 | 112.0 | 3.4 ± 1.1 | 565 ± 71 | 97.9 | 87.4 | 3.5 | 2.7 |
| Carrier 18 | 111.5 | 3.0 ± 0.01 | 90 ± 55 | 37.4 | 33.5 | 7.9 | 1.3 |

2.3. Influence of the Morphology of the Supports on the Hydrolytic Activities of the Biocatalysts

2.4. Influence of the Morphology of the Supports on the Esterification Activities of the Biocatalysts

2.5. Empirical Models
| Equation | |||||
|---|---|---|---|---|---|
| Ahid = a1 × Sesp + a3 × Vesp + b11 × Sesp × Sesp + b12 × Vesp × Dp + b13 × Sesp × Dp | |||||
| Estimated Parameters (R = 0.84; Degree of Freedom = 6) | |||||
| Parameters | a1 (U∙m−2) | a3 (106∙U∙m−3) | b11 (U∙g·m−4) | b12 (1016∙U∙m−4) | b13 (1010∙U∙m−3) |
| Estimated Values | 8.51 | −532.97 | −0.16 | 1.52 | −0.02 |
| Standard Errors | 2.20 | 239.72 | 0.03 | 0.65 | 0.01 |
| Significance | 0.992 | 0.932 | 0.999 | 0.942 | 0.986 |

| Equation | ||||
|---|---|---|---|---|
| Aester = a0 + a1 × Sesp + a2 × Dp + a3 × Vesp + b11 × Sesp × Dp + b12 × Vesp × Sesp + b13 × Vesp × Dp + c12 × Sesp × Dp × Vesp | ||||
| Estimated Parameters (R = 0.88; Degree of Freedom = 3) | ||||
| Parameters | Estimated Values | Standard Errors | Significance | |
| a0 | (U·g−1) | −19.5 | 1204.1 | 0.012 |
| a1 | (U·m−2) | 652.6 | 850.3 | 0.501 |
| a2 | (1010 U·(g·m)−1) | −0.2 | 3.6 | 0.032 |
| a3 | (106 U·m−3) | −39898.7 | 58152.5 | 0.458 |
| b11 | (1010 U·m−3) | −1.7 | 2.4 | 0.426 |
| b12 | (106 U·g·m−5) | −1977.8 | 7867.3 | 0.182 |
| b13 | (1016 U·m−4) | 147.3 | 148.8 | 0.605 |
| c12 | (1016 U·g·m−6) | 0.4 | 25.3 | 0.011 |

3. Experimental Section
3.1. Materials
3.2. Preparation of Core-Shell Polymer Supports
| Reaction | Legend | Graph Legend | Sesp (m2/g) | Vesp (cm3/g) | Dp (Å) |
|---|---|---|---|---|---|
| 1 1 | Floating Flow Rate | Floating Flow Rate | 27.3 | 0.20 | 287.6 |
| 2 2 | Core | Core | 0.2 | - | - |
| 3 | Reference; V = 0.035 L/h | V = 0.035 L/h | 7.8 | 0.06 | 300.9 |
| 4 3 | Reference; V = 0.039 L/h | V = 0.039 L/h | 2.2 | - | - |
| 5 3 | Reference; V = 0.071 L/h | V = 0.071 L/h | 5.7 | - | - |
| 6 4 | Reference; V = 0.127 L/h | V = 0.127 L/h | - | - | - |
| 7 3 | Reference; V = 0.082 L/h | V = 0.082 L/h | 1.4 | 0.01 | 416.4 |
| 8 | +25% Emulsifier; V = 0.035 L/h | +25% Emulsifier | 2.9 | 0.03 | 400.8 |
| 9 | −25% Emulsifier; V = 0.035 L/h | −25% Emulsifier | 6.5 | 0.05 | 341.4 |
| 10 | −25% Initiator; V = 0.035 L/h | −25% Initiator | 2.3 | 0.03 | 477.0 |
| 11 | +25% Initiator; V = 0.035 L/h | +25% Initiator | 11.2 | 0.08 | 299.7 |
| 12 | Reference; V = 0.017 L/h | V = 0.017 L/h | 3.3 | 0.02 | 327.6 |
| 13 | −25% Initiator; − 50% Monomer; V = 0.035 L/h | −Initiator; −Monomer | 5.3 | 0.04 | 333.0 |
| 14 | −25% Initiator; + 50% Monomer; V = 0.035 L/h | −Initiator; +Monomer | 13.1 | 0.09 | 263.7 |
| 15 | −50% Monomer; V = 0.035 L/h | 0 Initiator; −Monomer | 1.9 | 0.01 | 291.8 |
| 16 | +50% Monomer; V = 0.035 L/h | 0 Initiator; +Monomer | 4.3 | 0.03 | 300.4 |
| 17 | Reference; V = 0.035 L/h | 0 Initiator; 0 Monomer | 4.4 | 0.08 | 373.7 |
| 18 | −25% Initiator; V = 0.035 L/h | −Initiator; 0 Monomer | 1.7 | 0.01 | 306.9 |
| - | Commercial Support | Accurel | 39 | 0.24 | 230.0 |
3.3. Characterization of Supports Morphology

3.4. Immobilization Procedure
3.5. Hydrolytic Activity of Biocatalysts
3.6. Immobilization Parameters


3.7. Esterification Activity of Biocatalysts
3.8. Determination of Protein Concentration
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Freire, D.M.G.; Castilho, L.R. Lipases em biocatálise. In Enzimas Em Biotecnologia: Produção, Aplicações E Mercado, 1st ed.; Bon, E.P.S., Ferrara, M.A., Corvo, M.L., Eds.; Editora Interciência: Rio de Janeiro, Brazil, 2008; Volume 1, pp. 369–380. [Google Scholar]
- Cunha, A.G.; Besteti, M.D.; Manoel, E.A.; da Silva, A.A.T.; Almeida, R.V.; Simas, A.B.C.; Fernandez-Lafuente, R.; Pinto, J.C.; Freire, D.M.G. Preparation of core-shell polymer supports to immobilize lipase B from Candida Antarctica: Effect of the support nature on catalytic properties properties. J. Mol. Catal. B Enzym. 2014, 100, 59–67. [Google Scholar] [CrossRef]
- Forró, E.; Fülöp, F. Recent lipase-catalyzed hydrolytic approaches to pharmacologically important β - and γ -amino acids. Curr. Med. Chem. 2012, 19, 6178–6187. [Google Scholar] [CrossRef]
- Gotor-Fernández, V.; Brieva, R.; Gotor, V. Lipases: Useful biocatalysts for the preparation of pharmaceuticals. J. Mol. Catal. B Enzym. 2006, 40, 111–120. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R.; Armisén, P.; Sabuquillo, P.; Fernández-Lorente, G.; Guisán, J.M. Immobilization of lipases by selective adsorption on hydrophobic supports. Chem. Phys. Lipids. 1998, 93, 185–197. [Google Scholar]
- Villeneuve, P.; Muderhwa, J.M.; Graille, J.; Haas, M.J. Customizing lipases for biocatalysis: A survey of chemical, physical and molecular biological approaches. J. Mol. Catal. B Enzym. 2000, 9, 113–148. [Google Scholar] [CrossRef]
- Cammarota, M.C.; Freire, D.M.G. A review on hydrolytic enzymes in the treatment of wastewater with high oil and grease content. Bioresour. Technol. 2006, 97, 2195–2210. [Google Scholar] [CrossRef]
- Rigo, E.; Rigoni, R.E.; Lodea, P.; Oliveira, D.; Freire, D.M.G.; di Luccio, M. Application of different lipases as pretreatment in anaerobic treatment of wastewater. Environ. Eng. Sci. 2008, 25, 1243–1248. [Google Scholar] [CrossRef]
- Ribeiro, B.D.; Castro, A.M.; Coelho, M.A.Z.; Freire, D.M.G. Production and use of lipases in bioenergy: A review from the feedstocks to biodiesel production. Enzym. Res. 2011, 2011, 1–16. [Google Scholar]
- Ranganathan, S.V.; Narasimhan, S.L.; Muthukumar, K. An overview of enzymatic production of biodiesel. Bioresour. Technol. 2008, 99, 3975–3981. [Google Scholar] [CrossRef]
- Tan, T.; Lu, J.; Nie, K.; Deng, L.; Wang, F. Biodiesel production with immobilized lipase: A review. Biotechnol. Adv. 2010, 28, 628–634. [Google Scholar] [CrossRef]
- Castro, H.F.; Zanin, G.M.; Moraes, F.F.; Sá-Pereira, P. Imobilização de enzimas e sua estabilização. In Enzimas Em Biotecnologia: Produção, Aplicações E Mercado, 1st ed.; Bon, E.P.S., Ferrara, M.A., Corvo, M.L., Eds.; Editora Interciência: Rio de Janeiro, Brazil, 2008; Volume 1, pp. 123–151. [Google Scholar]
- Tischer, W.; Kasche, V. Immobilized enzymes: Crystals or carriers? Trends Biotechnol. 1999, 17, 326–335. [Google Scholar] [CrossRef]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef]
- Liese, A.; Hilterhaus, L. Evaluation of immobilized enzymes for industrial applications. Chem. Soc. Rev. 2013, 42, 6236–6249. [Google Scholar] [CrossRef]
- Stepankova, V.; Bidmanova, S.; Koudelakova, T.; Prokop, Z.; Chaloupkova, R.; Damborsky, J. Strategies for stabilization of enzymes in organic solvents. ACS Catal. 2013, 3, 2823–2836. [Google Scholar]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Verma, M.L.; Barrow, C.J.; Puri, M. Nanobiotechnology as a novel paradigm for enzyme immobilisation and stabilisation with potential applications in biodiesel production. Appl. Microbiol. Biotechnol. 2013, 97, 23–39. [Google Scholar] [CrossRef]
- Hwang, E.T.; Gu, M.B. Enzyme stabilization by nano/microsized hybrid materials. Eng. Life Sci. 2013, 13, 49–61. [Google Scholar]
- Garcia-Galan, C.; Berenguer-Murcia, A.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Cowan, D.A.; Fernandez-Lafuente, R. Enhancing the functional properties of thermophilic enzymes by chemical modification and immobilization. Enzyme Microb. Technol. 2011, 49, 326–346. [Google Scholar] [CrossRef]
- Emond, S.; Montanier, C.; Nicaud, J.M.; Marty, A.; Monsan, P.; André, I.; Remaud-Siméon, M. New efficient recombinant expression system to engineer Candida antarctica lipase B. Appl. Environ. Microbiol. 2010, 76, 2684–2687. [Google Scholar] [CrossRef]
- Barbosa, O.; Ruiz, M.; Ortiz, C.; Fernández, M.; Torres, R.; Fernandez-Lafuente, R. Modulation of the properties of immobilized CALB by chemical modification with 2,3,4-trinitrobenzenesulfonate or ethylendiamine: Advantages of using adsorbed lipases on hydrophobic supports. Process Biochem. 2012, 47, 867–876. [Google Scholar] [CrossRef]
- Uppenberg, J.; Öhmer, N.; Norin, M.; Hult, K.; Kleywegt, G.J.; Patkar, S.; Waagen, V.; Anthomen, T.; Jones, T.A. Crystallographic and molecular-modeling studies of lipase b from candida antarctica reveal a stereospecificity pocket for secondary alcohols. Biochemistry 1995, 34, 16838–16851. [Google Scholar] [CrossRef]
- Palomo, J.M.; Muñoz, G.; Fernández-Lorente, G.; Mateo, C.; Fernández-Lafuente, R.; Guisán, J.M. Interfacial adsorption of lipases on very hydrophobic support (octadecyl- Sepabeads): Immobilization, hyperactivation and stabilization of the open form of lipases. J. Mol. Catal. B Enzym. 2002, 19–20, 279–286. [Google Scholar] [CrossRef]
- Kennedy, J.T.; White, C.A. Principles of immobilization of enzymes. In Handbook of Enzyme Biotechnology, 2nd ed.; Wiseman, A., Ed.; John Wiley & Sons: New York, NY, USA, 1985; pp. 147–157. [Google Scholar]
- Kahraman, M.V.; Bayramoğlu, G.; Kayaman-Apohan, N.; Güngör, A. α-Amylase immobilization on functionalized glass beads by covalent attachment. Food Chem. 2007, 104, 1385–1392. [Google Scholar] [CrossRef]
- Pinto, J.C.; Alves, T.L.M.; Lima, E.L.; Salim, V.M.M.; Figueiredo, K.C.S.; Lenzi, M.K. PI 0400803–0. 30 March 2004.
- Besteti, M.D. Produção de Partículas Poliméricas Com Porosidade Controlada Para A Preparação de Biocatalisadores. Ph.D Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 2011. [Google Scholar]
- Figueiredo, K.C.S.; Salim, V.M.M.; Alves, T.L.M.; Pinto, J.C. Lysozyme Adsorption onto Different Supports: A Comparative Study. Adsorption 2005, 11, 131–138. [Google Scholar] [CrossRef]
- Besteti, M.D.; Cunha, A.G.; Freire, D.M.G.; Pinto, J.C. Core/Shell polymer particles by semibatch combined suspension/emulsion polymerizations for enzyme immobilization. Macromol. Mater. Eng. 2014, 299, 135–143. [Google Scholar]
- Manoel, E.A.; Pais, K.C.; Flores, M.C.; Miranda, L.S.M.; Coelho, M.A.Z.; Simas, A.B.C.; Freire, D.M.G.; de Souza, R.O.M.A. Kinetic resolution of a precursor for myo-inositol phosphates under continuous flow conditions. J. Mol. Catal. B Enzym. 2013, 87, 139–143. [Google Scholar] [CrossRef]
- Manoel, E.A.; Pais, K.C.; Cunha, A.G.; Coelho, M.A.Z.; Freire, D.M.G.; Simas, A.B.C. On the kinetic resolution of sterically hindered myo-inositol derivatives in organic media by lipases. Tetrahedron Asymmetry 2012, 23, 47–52. [Google Scholar] [CrossRef]
- Manoel, E.A.; Pais, K.C.; Cunha, A.G.; Simas, A.B.C.; Coelho, M.A.Z.; Freire, D.M.G. Kinetic resolution of 1,3,6-tri-O-benzyl-myo-inositol by Novozym 435: Optimization and enzyme reuse. Org. Process Res. Dev. 2012, 16, 1378–1384. [Google Scholar]
- Cunha, A.G.; da Silva, A.A.T.; da Silva, A.J.R.; Tinoco, L.W.; Almeida, R.V.; de Alencastro, R.B.; Simas, A.B.C.; Freire, D.M.G. Efficient kinetic resolution of (±)-1,2-O-benzyl-myo-inositol with the lipase B of Candida antarctica. Tetrahedron Asymmetry 2010, 21, 2899–2903. [Google Scholar] [CrossRef]
- Simas, A.B.C.; da Silva, A.A.T.; Cunha, A.G.; Assumpção, R.S.L.; Hoelz, V.B.; Neves, B.C.; Galvão, T.C.; Almeida, R.V.; Albuquerque, M.G.; Freire, D.M.G.; et al. Kinetic resolution of (±)-1,2-O-isopropylidene-3,6-di-O-benzyl-myo-inositol by lipases: An experimental and theoretical study on the reaction of a key precursor of chiral inositols. J. Mol. Catal. B Enzym. 2011, 70, 32–40. [Google Scholar] [CrossRef]
- Mojovic, L.; Knezevic, Z.; Popadic, R.; Jovanovic, S. Immobilization of lipase from Candida rugosa on a polymer support. Appl. Microbiol. Biotechnol. 1998, 50, 676–681. [Google Scholar] [CrossRef]
- Bayne, L.; Ulijn, R.V.; Halling, P.J. Effect of pore size on the performance of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 9000–9010. [Google Scholar] [CrossRef]
- Lenzi, M.K.; Silva, F.M.; Lima, E.L.; Pinto, J.C. Semibatch styrene suspension polymerization processes. J. Appl. Polym. Sci. 2003, 89, 3021–3038. [Google Scholar]
- Cunha, A.G. Resolução Cinética de Derivados de Mio-inositol Catalisada Por Lipases. Ph.D Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 2011. [Google Scholar]
- Cunha, A.G.; Fernández-Lorente, G.; Bevilaqua, J.V.; Destain, J.; Paiva, L.M.C.; Freire, D.M.G.; Fernández-Lafuente, R.; Guisán, J.M. Immobilization of Yarrowia lipolytica Lipase: A comparison of stability of physical adsorption and covalent attachment techniques. Appl. Biochem. Biotechnol. 2008, 146, 49–56. [Google Scholar]
- Cunha, A.G.; Fernández-Lorente, G.; Gutarra, M.L.E.; Bevilaqua, J.V.; Almeida, R.V.; Paiva, L.M.C.; Fernández-Lafuente, R.; Guisán, J.M.; Freire, D.M.G. Separation and immobilization of lipase from Penicillium simplicissimum by selective adsorption on hydrophobic supports. Appl. Biochem. Biotechnol. 2009, 156, 563–575. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sample Availability: Samples are not available, but can be produced for those who may be interested in the supports and biocatalysts.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pinto, M.C.C.; Freire, D.M.G.; Pinto, J.C. Influence of the Morphology of Core-Shell Supports on the Immobilization of Lipase B from Candida antarctica. Molecules 2014, 19, 12509-12530. https://doi.org/10.3390/molecules190812509
Pinto MCC, Freire DMG, Pinto JC. Influence of the Morphology of Core-Shell Supports on the Immobilization of Lipase B from Candida antarctica. Molecules. 2014; 19(8):12509-12530. https://doi.org/10.3390/molecules190812509
Chicago/Turabian StylePinto, Martina C. C., Denise M. G. Freire, and José Carlos Pinto. 2014. "Influence of the Morphology of Core-Shell Supports on the Immobilization of Lipase B from Candida antarctica" Molecules 19, no. 8: 12509-12530. https://doi.org/10.3390/molecules190812509
APA StylePinto, M. C. C., Freire, D. M. G., & Pinto, J. C. (2014). Influence of the Morphology of Core-Shell Supports on the Immobilization of Lipase B from Candida antarctica. Molecules, 19(8), 12509-12530. https://doi.org/10.3390/molecules190812509