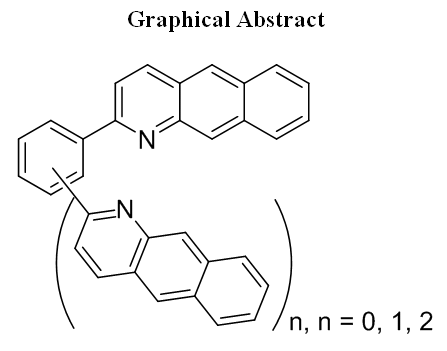
Unusual Product Distribution from Friedländer Reaction of Di- and Triacetylbenzenes with 3-Aminonaphthalene-2-carbaldehyde and Properties of New Benzo[g]quinoline-Derived Aza-aromatics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis

2.2. Spectroscopic Properties
| H2 of Ph | H3 of Ph | H2 of BQ | H3 of BQ | H4 of BQ | H5 of BQ | H10 of BQ | |
|---|---|---|---|---|---|---|---|
| 3aa | 8.24 | 7.89 | - | - | 8.38 | 8.40 | 8.77 |
| 3ab | - | 8.74 a | - | 8.57 | 8.46 | 8.44 | 8.79 |
| 3c | 9.12 | 8.40 | - | - | 8.46 | 8.43 | 8.70 |
| 3e | 9.30 | 8.25 | - | - | 8.52 | 8.48 | 8.90 |
| 4 | - | - | 8.97 | 7.35 | 8.58 | 8.40 | 8.76 |
| 5 | 8.51 | 8.26–8.15 | - | 8.26–8.15 | 8.689 | 8.69 | 8.81 |
| Compound | λabs/nm (logε) | λexcit | λem |
|---|---|---|---|
| 3aa | 205 (4.86) 228 (4.63) 257 (s, 4.60) 282 (4.91) 352 (3.81) 371 (3.96) | 282 | 481 |
| 3ab a | 204 (4.70) 225 (4.83) 259 (s, 4.78) 287 (4.98) 351 (4.04) 370 (4.15) | 225 | 488 |
| 3c | 205 (5.00) 228 (s, 4.80) 265 (s, 4.76) 286 (4.86) 371 (3.88) | 286 | 470 |
| 3e | 209 (s, 4.78) 216 (4.81) 249 (4.36) 294 (4.28) 378 (3.43) | 294 | 470 |
| 4 | 203 (4.30) 227 (4.46) 253 (4.88) 272 (s, 4.08) 358 (3.66) | 253 | 435 |
| 5 | 205 (4.83) 216 (4.88) 232 (s, 4.76) 246 (4.72) 298 (4.90) | 298 | 470 |

2.3. Thermal and Structural Properties


3. Experimental Section
General Information
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Evans, J.C.W.; Allen, C.F.H. 2-Phenylpyridine. Org. Syn. 1938, 18, 70. [Google Scholar] [CrossRef]
- Taiju, T.; Wei, H. Recent advances in multicolor emission and color tuning of heteroleptic iridium complexes. Isr. J. Chem. 2014, 54, 885–896. [Google Scholar] [CrossRef]
- Zhou, G.; Wong, W.-Y.; Yang, X. New design tactics in OLEDs using functionalized 2-phenylpyridine-type cyclometallates of iridium(III) and platinum(II). Chem. Asian J. 2011, 6, 1706–1727. [Google Scholar] [CrossRef] [PubMed]
- Tamayo, A.B.; Alleyne, B.D.; Djurovich, P.I.; Lamansky, S.; Tsyba, I.; Ho, N.N.; Bau, R.; Thompson, M.E. Synthesis and characterization of facial and meridional Tris-cyclometalated iridium(III) complexes. J. Am. Chem. Soc. 2003, 125, 7377–7387. [Google Scholar] [CrossRef] [PubMed]
- Tsuboyama, A.; Iwawaki, H.; Furugori, M.; Mukaide, T.; Kamatani, J.; Igawa, S.; Moriyama, T.; Miura, S.; Takiguchi, T.; Okada, S.; et al. Homoletptic cyclometalated iridium complexes with highly efficient red phosphorescence and application to organic light-emitting diode. J. Am. Chem. Soc. 2003, 125, 12971–12979. [Google Scholar]
- Baranoff, E.; Yum, J.-H.; Graetzel, M.; Nazeeruddin, M.K. Cyclometallated iridium complexes for conversion of light into electricity and electricity to light. J. Organomet. Chem. 2009, 694, 2661–2670. [Google Scholar] [CrossRef]
- Lowry, M.S.; Bernhard, S. Synthetically tailored excited states: Phosphorescent, cyclometalated iridium(III) complexes and their applications. Chem. Eur. J. 2006, 12, 7970–7977. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-H.; Cheng, Y.-M.; Chi, Y.; Hsu, C.-J.; Fang, F.-J.; Wong, K.-T.; Chou, P.-T.; Chang, C.-H.; Tsai, M.-H.; Wu, C.-C. Blue-emitting heteroleptic iridium(III) complexes suitable for high-efficiency phosphorescent OLEDs. Angew. Chem. Int. Ed. 2007, 46, 2418–2421. [Google Scholar] [CrossRef]
- Ulbricht, C.; Beyer, B.; Friebe, C.; Winter, A.; Schubert, U.S. Recent development in the application of phosphorescent iridium(III) complex systems. Adv. Mater. 2009, 21, 4418–4441. [Google Scholar] [CrossRef]
- Igarashi, T.; Kimura, K.; Nii, K. Light-Emitting Material Comprising Orthometalated Iridium Complex, Light-Emitting Device, High Efficiency Red Light-Emitting Device, and Novel Iridium Complex. U.S. Patent Application US20010019782, 6 September 2001. [Google Scholar]
- You, Y.; Huh, J.O.; Kim, K.S.; Lee, S.W.; Kim, D.; Park, S.Y. Comment on “aggregation-induced phosphorescent emission (AIPE) of iridium(III) complexes”: Origin of the enhanced phosphorescence. Chem. Commun. 2008, 3998–4000. [Google Scholar] [CrossRef]
- Adachi, C.; Baldo, M.A.; Thompson, M.E.; Forrest, S.R. Nearly 100% internal phosphorescence efficiency in an organic light-emitting device. J. Appl. Phys. 2001, 90, 5048–5051. [Google Scholar] [CrossRef]
- Wu, H.; Yang, T.; Zhao, Q.; Zhou, J.; Li, C.; Li, F. A cyclometalated iridium(III) complex with enhanced phosphorescence emission in the solid state (EPESS): Synthesis, characterization and its application in bioimaging. J. Chem. Soc. Dalton Trans. 2011, 40, 1969–1976. [Google Scholar] [CrossRef]
- Chirdon, D.N.; Transue, W.J.; Kagalwala, H.N.; Kaur, A.; Maurer, A.B.; Pintauer, T.; Bernhard, S. [Ir(N^N^N)(C^N)L]+: A new family of luminophores combining tunability and enhanced photostability. Inorg. Chem. 2014, 53, 1487–1499. [Google Scholar] [CrossRef] [PubMed]
- Lamansky, S.; Djurovich, P.; Murphy, D.; Abdel-Razzaq, F.; Kwong, R.; Tsyba, I.; Bortz, M.; Mui, B.; Bau, R.; Thompson, M.E. Synthesis and characterization of phosphorescent cyclometalated iridium complexes. Inorg. Chem. 2001, 40, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Shin, I.-S.; Kim, J.I.; Kwon, T.-H.; Hong, J.-I.; Lee, J.-K.; Kim, A. Efficient electrogenerated chemiluminescence from bis-cyclometalated iridium(III) complexes with substituted 2-phenylquinoline ligands. J. Phys. Chem. 2007, 111, 2280–2286. [Google Scholar]
- Qiao, J.; Duan, L.; Tang, L.; He, L.; Wang, L.; Qiu, Y. High-efficiey orange to near-infrared emissions from bis-cyclometalated iridium complexes with phenyl-benzoquinoline isomers as ligands. J. Mater. Chem. 2009, 19, 6573–6580. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, H.; Gao, Y.; Tao, R.; Xin, L.; Yi, J.; Li, F.; Liu, W.; Qiao, J. Near-infrared-emitting iridium(III) complexes as phosphorescent dyes for living cell imaging. Organometallics 2014, 33, 61–68. [Google Scholar] [CrossRef]
- Etienne, A. Action of phenylmagnesium bromide on 1-azaanthracene. Comptes Rend. 1944, 219, 622–624. [Google Scholar]
- Bergstrom, F.W.; McCallister, S.H. The preparation of 2-alkyl and 2-aryl pyridines and quinolines by the Grignard reaction. J. Am. Chem. Soc. 1930, 52, 2845–2849. [Google Scholar] [CrossRef]
- Staehelin, A. Synthesis of α-azaanthracenes starting from linear benzisatin. Comptes Rend. 1951, 233, 262–264. [Google Scholar]
- Yao, M.; Inoue, H.; Yoshioka, N. Novel aromatic N-oxyl radical based on the benzo[g]quinoline skeleton: Synthesis and intermolecular ferromagnetic interaction. Chem. Phys. Lett. 2005, 402, 11–16. [Google Scholar] [CrossRef]
- Huo, Z.; Gridnev, I.D.; Yamamoto, Y. A method for the synthesis of substituted quinolones via electrophilic cyclization of 1-azido-2-(2-propynyl)benzenes. J. Org. Chem. 2010, 75, 1266–1270. [Google Scholar] [CrossRef] [PubMed]
- Taffarel, E.; Chirayil, S.; Thummel, R.P. Synthesis and properties of ligands based on benzo[g]quinoline. J. Org. Chem. 1994, 59, 823–828. [Google Scholar] [CrossRef]
- Liang, J.; Cha, H.; Jahng, Y. Synthesis and properties of annulated 2-(azaar-2-yl)-and 2,2'-di(azar-2-yl)-9,9'-spirobifluorens. Molecules 2013, 18, 13680–13690, and references therein. [Google Scholar]
- Haginiwa, J.; Higuchi, Y.; Ikeda, S. Reactions concerned in tertiary amine N-oxides XIII. reactions of benzo[f, h and g]quinoline and acridine with aromatic amine N-oxides. Yakugaku Zasshi 1979, 99, 1181–1185. [Google Scholar] in which 3ab was prepared by a reaction of benzo[g]quinoline and pyridine-N-oxide in 0.3% yield.
- Krapcho, A.P.; Gilmor, T.P. General preparative route to benzo[g]quinoline (1-azaanthracenes). J. Heterocycl. Chem. 1999, 36, 445–452. [Google Scholar] [CrossRef]
- Rahman, A.F.M.M.; Jahng, Y. Synthesis and properties of benzo[b]-1,10-phenanthrolines and their ruthenium(II) complexes. Heteroat. Chem. 2007, 18, 650–656. [Google Scholar] [CrossRef]
- Rahman, A.F.M.M.; Kwon, Y.; Jahng, Y. Friedländer reactions of triacetylmethane: Unusual distribution of products. Heterocycles 2005, 65, 2777–2782. [Google Scholar] [CrossRef]
- Huu-Hoi, N.P.; Perin, F.; Jacquignon, P. Nitrogen heterocyclic analogs of polyaryls. J. Heterocycl. Chem 1965, 2, 7–10. [Google Scholar]
- Sample Availability: Samples of the compounds 3aa, 3ab, 3c, 3e, 4, and 5 are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Karim, M.; Jahng, Y. Unusual Product Distribution from Friedländer Reaction of Di- and Triacetylbenzenes with 3-Aminonaphthalene-2-carbaldehyde and Properties of New Benzo[g]quinoline-Derived Aza-aromatics. Molecules 2014, 19, 12842-12851. https://doi.org/10.3390/molecules190812842
Karim M, Jahng Y. Unusual Product Distribution from Friedländer Reaction of Di- and Triacetylbenzenes with 3-Aminonaphthalene-2-carbaldehyde and Properties of New Benzo[g]quinoline-Derived Aza-aromatics. Molecules. 2014; 19(8):12842-12851. https://doi.org/10.3390/molecules190812842
Chicago/Turabian StyleKarim, Moinul, and Yurngdong Jahng. 2014. "Unusual Product Distribution from Friedländer Reaction of Di- and Triacetylbenzenes with 3-Aminonaphthalene-2-carbaldehyde and Properties of New Benzo[g]quinoline-Derived Aza-aromatics" Molecules 19, no. 8: 12842-12851. https://doi.org/10.3390/molecules190812842
APA StyleKarim, M., & Jahng, Y. (2014). Unusual Product Distribution from Friedländer Reaction of Di- and Triacetylbenzenes with 3-Aminonaphthalene-2-carbaldehyde and Properties of New Benzo[g]quinoline-Derived Aza-aromatics. Molecules, 19(8), 12842-12851. https://doi.org/10.3390/molecules190812842