Phenolics and Polyphenolics from Melastomataceae Species
Abstract
:1. Introduction
2. Chemical Constituents of Melastomataceous Plants
2.1. Triterpenoids and Alkyl Benzoquinones

2.2. Flavonoids




2.3. Anthocyanins and Anthocyanidins
2.4. Phenolic Acids and Derivatives
2.5. Galloylated Cyanogenic Glucosides and Benzyl Glycosides

2.6. Hydrolyzable Tannins
2.6.1. Galloyl Glycosides


2.6.2. Ellagitannins







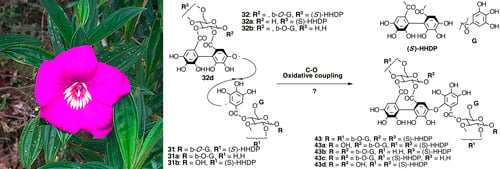
2.6.3. Biogenesis of Ellagitannins in Melastomataceous Plants
Biogenesis of Monomeric Hydrolyzable Tannins in Melastomataceae

| n | Pterocaryanin C-Type | |||
|---|---|---|---|---|
| Pterocaryanin C (31) | Praecoxin B (31b) (α/β) | 1,4,6-tri-O-Galloylglycopyranose (31a) | Nobotanin D (30) | |
| 1H-NMR | ||||
| H-1 | 6.19 d (8.0) | 5.48 d (3.5)/5.17 d (8) | 5.80 d (8.5) | 6.17 d (8) |
| H-2 | 5.14 dd (8.0, 1.0) | 5.07 dd (9.5, 3.5)/4.88 dd (8, 9.5) | 3.71 dd (8.0, 9.0) | 5.05 dd (8, 10) |
| H-3 | 5.44 t (10) | 5.62 t (9.5)/5.35 t (9.5) | 3.96 t (9.0) | 5.24 t (10) |
| H-4 | 5.58 t (10) | 5.50 t (10)/5.46 t (9.5) | 5.24 t (9.0) | 3.98 t (10) |
| H-5 | 4.17 dd (3.0, 1.0) | 4.54 ddd (10, 4, 2)/4.22 ddd (9.5, 5, 2) | 4.14 m | 4.07 m |
| Ha-6/Hb-6 | 4.46 br d (13)/4.24 dd (13.0, 13) | 4.49 dd (12, 2)/4.27 dd (12, 4)/4.26 d (12, 5) | 4.47 dd (14.0, 3.5) 4.14 m | 4.60 dd (1.5, 12)/4.45 dd (5, 12) |
| Galloyl | 7.16, 7.15, 7.13 (s) | 7.142/7.139/7.13/7.10 | 6.92, 6.95, 7.02 (s) | 7.12, 7.11, 2H (s) |
| 2,3-HHDP | β1,4,6 (S) | 4,6 (S)/6.60/6.59, 6.39 | 6.70, 6.42, β1,6 (S) | |
| Ref. | [28,42,80,82,83,84] | [79] | [51,84] | [28] |
| n | Casuarictin-Type | |||
|---|---|---|---|---|
| Casuarictin (32) | Pedunculagin (32a) (α/β) | Strictinin (32b) | Isostrictinin (32c) | |
| 1H-NMR | ||||
| H-1 | 6.21 d (8.5) | 5.43 d (3, 5)/5.22 d (9) | 5.76 d (7) | 6.08 d (8.5) |
| H-2 | 5.0 dd (8, 9) | 4.83 dd (8.5, 9.5) | 3.66 dd (7, 9) | 4.99 dd (8.5, 9.0) |
| H-3 | 5.49 t (9.0) | 5.44 t (10)/5.20 t (10.0) | 3.84 t (9) | 5.17 t (9.5) |
| H-4 | 4.99 t (10) | 4.91 t (9) | 3.88 t (9.5) | |
| H-5 | 4.58 dd (5.7, 9) | 4.18 ddd (10.0, 6.5, 1.5)/4.57 ddd (10.0, 7.0, 1.5) | 4.11 dd (6, 9) | 3.71 ddd (9.5, 5.5, 2.0) |
| Ha-6/Hb-6 | 3.84 d (13)/5.12 dd (6.5, 13) | 5.25 dd (13.0, 6.5)/5.22 dd (13.0, 7.0)/3.82 dd (13.0, 1.5)/3.76 dd (13.0, 1.5) | 5.22 dd (6, 14)/3.79 d (14) | 3.87 d (12)/3.78 dd (12, 5.5) |
| Galloyl | 7.18 (s) 2-H | ---- | 7.16 (s) 2-H | 7.12 (s) 2-H |
| 2,3-HHDP | 6.68, 6.55, β1 S | 6.66/6.64, 6.60/6.59, S | 6.70, 6.41 | |
| 4,6-HHDP | 6.47, 6.38, β1 S | 6.55/6.50, 6.33/6.32, S | 6.72, 6.61, β1 S | |
| Ref. | [22] | [85,86] | [84,85] | [84,85] |
| n | Pterocaryanin C-Type | |||
|---|---|---|---|---|
| Pterocaryanin C (31) | Praecoxin B (31b) (α/β) | 1,4,6-Tri-O-galloylglycopyranose (31a) | Nobotanin D (30) | |
| 13C-NMR | ||||
| C-1 | 91.9 | 91.3/94.9 | 95.4 | 92.1 |
| C-2 | 75.3 | 75.1/77.7 | 73.7 | 79.9 |
| C-3 | 77.4 | 75.4/77.6 | 75.2 | 76.2 |
| C-4 | 67.8 | 68.7/68.5 | 71.5 | 75.3 |
| C-5 | 73.9 | 68.4/73.1 | 73.7 | 67.8 |
| C-6 | 62.7 | 63.1/63.2 | 63.3 | 63.6 |
| ESI-MS | 937 [M − H]− | 785 [M − H]− | 635 [M − H]− | 786 [M − H]− |
| Ref. | [28,42,80,82] | [28,42,82,89,90] | [42,90] | [28] |
| n | Casuarictin-Type | |||
|---|---|---|---|---|
| Casuarictin (32) | Pedunculagin (32a) (α/β) | Strictinin (32b) | Isostrictinin (32c) | |
| 13C-NMR | ||||
| C-1 | 92.4 | 91.8 | 95.9 | 92.9 |
| C-2 | 76.0 | 75.6 | 74.7 | 71.1 |
| C-3 | 77.3 | 75.8 | 75.6 | 72.9 |
| C-4 | 69.3 | 69.9 | 72.8 | 73.4 |
| C-5 | 73.5 | 73.4 | 73.2 | 69.2 |
| C-6 | 63.1 | 63.8 | 63.7 | 65.9 |
| ESI-MS | 935 [M − H]− | 783 [M − H]− | 633(100) [M − H]− | 633(100) [M − H]− |
| Ref. | [22,64,82,84,91,92] | [82,84,92] | [64,82,84,90,92] | [64,82,84,92] |

Oligomeric Ellagitannins

Biogenesis of Oligomeric Ellagitannins in Melastomataceae
Biogenesis of Dimeric Hydrolyzable Tannins
Biogenesis of Trimeric Hydrolyzable Tannins

Biogenesis of Tetrameric Hydrolyzable Tannins

Biogenesis of Melastoflorins A–E


2.7. Condensed Tannins
3. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Michelangeli, F.A.; Guimaraes, P.J.F.; Penneys, D.S.; Almeda, F.; Kriebel, R. Phylogenetic relationships and distribution of new world melastomeae (melastomataceae). Bot. J. Linn. Soc. 2013, 171, 38–60. [Google Scholar] [CrossRef]
- Renner, S.S. Phylogeny and classification of the Melastomataceae and memecylaceae. Nord. J. Bot. 1993, 13, 519–593. [Google Scholar] [CrossRef]
- Kriebel, R.; Michelangeli, F.A.; Kelly, L.M. Discovery of unusual anatomical and continuous characters in the evolutionary history of Conostegia (Miconieae: Melastomataceae). Mol. Phylogenet. Evol. 2015, 82, 289–313. [Google Scholar] [CrossRef] [PubMed]
- Ocampo, G.; Michelangeli, F.A.; Almeda, F. Seed diversity in the tribe Miconieae (Melastomataceae): Taxonomic, systematic, and evolutionary implications. PLoS ONE 2014, 9. [Google Scholar] [CrossRef]
- Penneys, D.S. Preliminary phylogeny of the Astronieae (Melastomataceae) based on nuclear and plastid DNA sequence data, With comments on the Philippine endemic genus, Astrocalyx. Philipp. J. Sci. 2013, 142, 159–168. [Google Scholar]
- Goldenberg, R.; Baumgratz, J.F.A.; Souza, M.L.D.R. Taxonomy of Melastomataceae in Brazil: Retrospective and perspective views, and an identification key for the genera. Rodriguesia 2012, 63, 145–161. [Google Scholar] [CrossRef]
- Alice Boyle, W.; Bronstein, J.L. Phenology of tropical understory trees: Patterns and correlates. Rev. Biol. Trop. 2012, 60, 1415–1429. [Google Scholar]
- Chan, W.R.; Sheppard, V.; Medford, K.; Tinto, W.F.; Reynolds, W.F.; McLean, S. Triterpenes from Miconia stenotachya. J. Nat. Prod. 1992, 55, 963–966. [Google Scholar] [CrossRef]
- Kingston, D.G.; Abdel-Kader, M.; Zhou, B.-N.; Yang, S.-W.; Berger, J.M.; van der Werff, H.; Evans, R.; Mittermeier, R.; Malone, S.; Famolare, L. Biodiversity conservation, economic development, and drug discovery in Suriname. Biol. Act. Nat. Prod. Pharm. 2000, 39–49. [Google Scholar]
- Ndjateu, F.S.T.; Tsafack, R.B.N.; Nganou, B.K.; Awouafack, M.D.; Wabo, H.K.; Tene, M.; Tane, P.; Eloff, J.N. Antimicrobial and antioxidant activities of extracts and ten compounds from three Cameroonian medicinal plants: Dissotis perkinsiae (Melastomaceae), Adenocarpus mannii (Fabaceae) and Barteria fistulosa (Passifloraceae). S. Afr. J. Bot. 2014, 91, 37–42. [Google Scholar] [CrossRef]
- Dos Santos, F.M.; de Souza, M.G.; Crotti, A.E.M.; Martins, C.H.G.; Ambrósio, S.R.; Veneziani, R.C.S.; e Silva, M.L.A.; Cunha, W.R. Evaluation of antimicrobial activity of extracts of Tibouchina candolleana (melastomataceae), isolated compounds and semi-synthetic derivatives against endodontic bacteria. Braz. J. Microbiol. 2012, 43, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Hegnauer, R. Chemotaxonomie der Pflanzen; Birkhäuser Verlag Basel: Basilea, Switzerland, 1969; Volume V, p. 506. [Google Scholar]
- Isaza M., J.H.; Veloza C., L.A.; Ramírez A., L.S.; Tapias, J.; A., C.; Maya, D.C.; Díaz G., S.J. Flavonoides del extracto isopropanol-agua de Tibouchina ciliaris (melastomataceae). Sci. Tech. 2007, 1, 355–357. [Google Scholar]
- Harbone, J.B. Plant Polyphenols- XI. The structure of acilated anthocyanins. Phytochemistry 1964, 3, 151–160. [Google Scholar] [CrossRef]
- Bennini, B.; Chulia, A.J.; Kaouadji, M.; Thomasson, F. Flavonoid Glycosides from Erica cinerea. Phytochemistry 1992, 31, 2483–2486. [Google Scholar]
- Barbera, O.; Sanz, J.F.; Sanchez-Parareda, J.; Marco, J.A. Futher Flavonol Glycosides from Anthyllis onobrychioides. Phytochemistry 1986, 25, 2361–2365. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Wang, K.-J. Studies on the constituents of Pittosporum pentandrum. J. Taiwan Pharm. Assoc. 1987, 39, 33–39. [Google Scholar]
- Shigematsu, N.; Kouno, I.; Kawano, N. Quercetin 3-(6″-caffeoylgalactoside) from Hydrocotyle sibthorpioides. Phytochemistry 1982, 21, 2156–2158. [Google Scholar] [CrossRef]
- Isaza, J.H.; Ito, H.; Yoshida, T. A flavonol glycoside-lignan ester and accompanying acylated glucosides from Monochaetum multiflorum. Phytochemistry 2001, 58, 321–327. [Google Scholar] [CrossRef]
- Park, S.-H.; Oh, S.R.; Jung, K.Y.; Lee, I.S.; Ahh, K.S.; Kim, J.H.; Kim, Y.S.; Lee, H.-K. Acylated flavonol Glycosides with Anti-complement Activity from Persicaria lapathifolia. Chem. Pharm. Bull. 1999, 47, 1484–1485. [Google Scholar] [CrossRef] [PubMed]
- Mimura, M.R.M.; Salatino, A.; Salatino, M.L.F. Distribution of flavonoids and the taxonomy of Huberia (Melastomataceae). Biochem. Syst. Ecol. 2004, 32, 27–34. [Google Scholar] [CrossRef]
- Rodrigues, J.; Rinaldo, D.; da Silva, M.A.; dos Santos, L.C.; Vilegas, W. Secondary metabolites of Miconia rubiginosa. J. Med. Food 2011, 14, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.; Schadow, B.; Quijano, C.E.; Marx, F. Chemical characterization and antioxidant capacity of berries from Clidemia rubra (Aubl.) Mart. (Melastomataceae). Food Res. Int. 2011, 44, 2120–2127. [Google Scholar] [CrossRef]
- Tarawneh, A.H.; León, F.; Ibrahim, M.A.; Pettaway, S.; McCurdy, C.R.; Cutler, S.J. Flavanones from Miconia prasina. Phytochem. Lett. 2014, 7, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Bomfim-Patrício, M.C.; Salatino, A.; Martins, A.B.; Wurdack, J.J.; Salatino, M.L.F. Flavonoids of Lavoisiera, Microlicia and Trembleya (Melastomataceae) and their taxonomic meaning. Biochem. Syst. Ecol. 2001, 29, 711–726. [Google Scholar] [CrossRef]
- Rodrigues, J.; Rinaldo, D.; dos Santos, L.C.; Vilegas, W. An unusual linked flavonoid from Miconia cabucu (Melastomataceae). Phytochemistry 2007, 68, 1781–1784. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. Plant Plliphenols-XI. The estructure of Acylated Anthocyanins. Phytochemistry 1964, 3, 151–160. [Google Scholar] [CrossRef]
- Yoshida, T.; Ohbayashi, H.; Ishihara, K.; Ohwashi, W.; Haba, K.; Okano, Y.; Shingu, T.; Okuda, T. Tannins and related polyphenols of Melstomataceous Plants I. Hydrolyzable Tannins from Tibouchina semidecandra Cogn. Chem. Pharm. Bull. 1991, 39, 2233–2240. [Google Scholar] [CrossRef]
- Yoshida, T.; Nakata, F.; Hosotani, K.; Nitta, A.; Okudat, T. Dimeric hydrolysable tannins from Melastoma malabathricum. Phytochemistry 1992, 31, 2829–2833. [Google Scholar] [CrossRef]
- Yoshida, H.; Arioka, H.; Fujita, T.; Chen, X.-H.; Okuda, T. Monomeric and dimeric hydrolyzable tannins from two Melastomataceous species. Phytochemistry 1994, 37, 863–866. [Google Scholar] [CrossRef]
- Ishii, R.; Saito, K.; Horie, M.; Shibano, T.; Kitanaka, S.; Amano, F. Inhibitory effects of hydrolyzable tannins from Melastoma dodecandrum Lour. on nitric oxide production by a murine macrophage-like cell line, RAW264. 7, activated with lipopolysaccharide and interferon-gamma. Biol. Pharm. Bull. 1999, 22, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Sirat, H.M.; Rezali, M.F.; Ujang, Z. Isolation and Identification of Radical Scavenging and Tyrosinase Inhibition of Polyphenols from Tibouchina semidecandra L. J. Agric. Food. Chem. 2010, 58, 10404–10409. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B.; Baxter, H. The Handbook of Natural Flavonoids; John Wiley & Sons: Chichester, UK, 1999; Volume 1, p. 889. [Google Scholar]
- Anuar, N.; Mohd Adnan, A.F.; Saat, N.; Aziz, N.; Mat Taha, R. Optimization of extraction parameters by using response surface methodology, purification, and identification of anthocyanin pigments in Melastoma malabathricum fruit. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Francis, F.J.; Baldini, V.; Laderoza, M. New anthocyanins from Tibouchina granulosa. J. Am. Soc. Hortic. Sci 1982, 107, 789–791. [Google Scholar]
- Bobbio, F.O.; Bobbio, P.A.; Degáspari, C.H. Anthocyanins from Tibouchina grandiflora. Food Chem. 1985, 18, 153–159. [Google Scholar] [CrossRef]
- Terahara, N.; Susuki, H.; Toki, K.; Kuwano, H.; Saito, N.; Honda, T. A diacylated anthocyanin from Tibouchina urvilleana Flowers. J. Nat. Prod. 1993, 56, 335–340. [Google Scholar] [CrossRef]
- Janna, O.A.; Khairul, A.; Maziah, M.; Mohd, Y. Flower pigment analysis of Melastoma malabathricum. Afr. J. Biotechnol. 2006, 5, 170–174. [Google Scholar]
- Janna, O.A.; Khairul, A.K.; Maziah, M. Anthocyanin stability studies in Tibouchina semidecandra L. Food Chem. 2007, 101, 1640–1646. [Google Scholar] [CrossRef]
- Singh, L.K.; Karlo, T.; Pandey, A. Performance of fruit extract of Melastoma malabathricum L. as sensitizer in DSSCs. Spectrochim. Acta Part A 2014, 118, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Bate-Smith, E.C. The phenolic constituents of plants and their taxonomic significance. J. Linn. Soc. Lond. Bot. 1962, 58, 95–114. [Google Scholar] [CrossRef]
- Hooi Poay, T.; Sui Kiong, L.; Cheng Hock, C. Characterisation of galloylated cyanogenic glucosides and hydrolysable tannins from leaves of Phyllagathis rotundifolia by LC-ESI-MS/MS. Phytochem. Anal. 2011, 22, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.-K.; Tanaka, T.; Kouno, I. New Cyanogenic and Alkyl Glycoside Constituents from Phyllagathis rotundifolia. J. Nat. Prod. 2002, 65, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.E.; Stewart, M.; Capon, R.J.; Woodrow, I.E. A galloylated cyanogenic glycoside from the Australian endemic rainforest tree Elaeocarpus sericopetalus (Elaeocarpaceae). Phytochemistry 2006, 67, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Franks, T.K.; Hayasaka, Y.; Choimes, S.; van Heeswijck, R. Cyanogenic glucosides in grapevine: polymorphism, identification and developmental patterns. Phytochemistry 2005, 66, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Isaza M., J.H.; Veloza, L.A.; Ramirez, L.S.; Guevara, C.A. Estimación espectrofométrica de taninos hidrolizables y condensados en plantas Melastomatáceas. Sci. Tech. Año XIII 2007, 1, 261–266. [Google Scholar]
- Isaza M., J.H.; Veloza, L.A.; Guevara, C.A.; Avila, Y.P.; DÌaz, O. Estimación espectrofotometrica de fenoles totales en especies de la familia Melastomataceae. Actual. Biol. 2005, 27, 75–79. [Google Scholar]
- Nokara, G.-I.; Nisthioka, I. Tannins and related compounds. X. Rhubarb (2): Isolation and structures of a glycerol gallate, gallic acid glucoside gallates, galloylglucoses and isolindleyin. Chem. Pharm. Bull. 1983, 31, 1652–1658. [Google Scholar]
- He, Q.; Shi, B.; Yao, K.; Luo, Y.; Ma, Z. Synthesis of gallotannins. Carbohydr. Res. 2001, 335, 245–250. [Google Scholar] [CrossRef]
- Lee, S.-H.; Tanaka, T.; Nonaka, G.-I.; Nishioka, I. Hydrolysable tannins from Euphorbia thymifolia. Phytochemistry 1990, 29, 3621–3625. [Google Scholar] [CrossRef]
- Nawwar, M.A.M.; Huussein, S.A.M.; Merfort, I. NMR Spectral Analysis of Polyphenols from Punica granatum. Phytochemistry 1994, 36, 793–798. [Google Scholar] [CrossRef]
- Lee, M.-W.; Tanaka, T.; Nonaka, G.-I.; Nishioka, I. Hirsunin, an ellagitannin with a diarylheptanoid moiety, from Alnus hirsuta var. Microphylla. Phytochemistry 1992, 31, 967–970. [Google Scholar]
- Nonaka, G.; Sakai, R.; Nishioka, I. Hydrolyzable Tannins and poanthocianidins from green Tea. Phytochemistry 1984, 23, 1753–1755. [Google Scholar] [CrossRef]
- Lee Ho, S.; Tanaka, T.; Nonaka, G.-I.; Nishioka, I. Sedoheptulose digallate from Cornus officinalis. Phytochemistry 1989, 28, 3469–3472. [Google Scholar] [CrossRef]
- Lee, S.-H.; Tanaka, T.; Nonaka, G.-I.; Nishioka, I.; Zhang, B. Allose gallates from Euphorbia fischeriana. Phytochemistry 1991, 30, 1251–1253. [Google Scholar] [CrossRef]
- Wilkins, C. Galloyl glucose derivatives from Heuchera cylindrica. Phytochemistry 1988, 27, 2317–2318. [Google Scholar] [CrossRef]
- Duan, D.; Li, Z.; Luo, H.; Zhang, W.; Chen, L.; Xu, X. Antiviral compounds from traditional Chinese medicines Galla Chinese as inhibitors of HCV NS3 protease. Bioorg. Med. Chem. Lett. 2004, 14, 6041–6044. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Huang, Y.-N.; Xu, P.-Y.; Kawabata, J. Inhibitory effect on α-glucosidase by the fruits of Terminalia chebula Retz. Food Chem. 2007, 105, 628–634. [Google Scholar] [CrossRef]
- Meyers, K.J.; Swiecki, T.J.; Mitchell, A.E. Understanding the native Californian diet: Identification of condensed and hydrolyzable tannins in tanoak acorns (Lithocarpus densiflorus). J. Agric. Food. Chem. 2006, 54, 7686–7691. [Google Scholar] [CrossRef] [PubMed]
- Salminen, J.-P.; Ossipov, V.; Haukioja, E.; Pihlaja, K. Seasonal variation in the content of hydrolysable tannins in leaves of Betula pubescens. Phytochemistry 2001, 57, 15–22. [Google Scholar] [CrossRef]
- Soong, Y.Y.; Barlow, P.J. Isolation and structure elucidation of phenolic compounds from longan (Dimocarpus longan Lour.) seed by high-performance liquid chromatography—Electrospray ionization mass spectrometry. J. Chromatogr. A 2005, 1085, 270–277. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Xia, W. Analysis of phenolic compounds in Chinese olive (Canarium album L.) fruit by RPHPLC-DAD-ESI-MS. Food Chem. 2007, 105, 1307–1311. [Google Scholar] [CrossRef]
- Kool, M.M.; Comeskey, D.J.; Cooney, J.M.; McGhie, T.K. Structural identification of the main ellagitannins of a boysenberry (Rubus loganbaccus × baileyanus Britt.) extract by LC–ESI-MS/MS, MALDI-TOF-MS and NMR spectroscopy. Food Chem. 2010, 119, 1535–1543. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Schmeda-Hirschmann, G. Determination of phenolic composition and antioxidant activity in fruits, rhizomes and leaves of the white strawberry (Fragaria chiloensis spp. chiloensis form chiloensis) using HPLC-DAD-ESI-MS and free radical quenching techniques. J. Food Compos. Anal. 2010, 23, 545–553. [Google Scholar] [CrossRef]
- Mämmelä, P.; Savolainen, H.; Lindroos, L.; Kangas, J.; Vartiainen, T. Analysis of oak tannins by liquid chromatography-electrospray ionisation mass spectrometry. J. Chromatogr. A 2000, 891, 75–83. [Google Scholar] [CrossRef]
- Zywicki, B.; Reemtsma, T.; Jekel, M. Analysis of commercial vegetable tanning agents by reversed-phase liquid chromatography—Electrospray ionization–tandem mass spectrometry and its application to wastewater. J. Chromatogr. A 2002, 970, 191–200. [Google Scholar] [CrossRef]
- Yoshida, T.; Amakura, Y.; Yoshimura, M. Structural Features and Biological Properties of Ellagitannins in Some Plant Families of the Order Myrtales. Int. J. Mol. Sci. 2010, 11, 79–106. [Google Scholar] [CrossRef] [PubMed]
- Lowry, J.B. The distribution and potential taxonomic value of alkylated ellagic acids. Phytochemistry 1968, 7, 1803–1813. [Google Scholar] [CrossRef]
- Yoshida, T.; Ikeda, Y.; Ishihara, K.; Ohwaski, W.; Shingu, T.; Okuda, T. Dimeric ellagitannins in plants of Melastomataceae. Chem. Pharm. Bull. 1986, 34, 2676–2679. [Google Scholar] [CrossRef]
- Yoshida, H.; Haba, K.; Shingu, T.; Okuda, T. Revised structure of Nobotanin B, a dimeric ellagitannin of Tibouchina semidecandra. Heterocycles 1987, 26, 2845–2848. [Google Scholar]
- Yoshida, T.; Ohwashi, W.; Haba, K.; Ohbayashi, H.; Ishihara, K.; Okano, Y.; Shingu, T.; Okuda, T. Tannins and Related Polyphenols of Melastomataceous Plants. II. Nobotanins B, C and E, Hydrolyzable Tannin Dimer and Trimers from Tibouchina semidecandra COGN. Chem. Pharm. Bull. 1991, 39, 2264–2270. [Google Scholar] [CrossRef]
- Yoshida, T.; Hatano, T.; Ito, H.; Okuda, T. Chemical and biological perspectives of ellagitannin oligomers from medicinal plants. In Studies in Natural Products Chemistry; Atta ur, R., Ed.; Elsevier: Okayama, Japan, 2000; Volume 23, pp. 395–453. [Google Scholar]
- Niemetz, R.; Niehaus, J.U.; Gross, G.G. Biosynthesis and Biodegradation of Complex Gallotannins. In Plant Polyphenols 2: Chemistry, Biology, Pharmacology, Ecology; Gross, G.G., Hemingway, R.W., Yoshida, T., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999; pp. 63–82. [Google Scholar]
- Gross, G.G. Enzymes in the Biosynthesis of Hydrolyzable Tannins. In Plant Polyphenols; Hemingway, R.W., Laks, P.E, Eds.; Springer US/Plenum Press: New York, NY, USA, 1992; pp. 43–60. [Google Scholar]
- Haslam, E. The Metabolism of Gallic Acid and Hexahydroxydiphenic Acid in Higher Plants. In Progress in Chemistry of Organic Natural Products; Berlin, S., Ed.; Springer: Sheffield, UK, 1982; Volume 41, pp. 1–46. [Google Scholar]
- Helm, R.F.; Zhentian, L.; Ranatunga, T.; Jervis, J.; Elder, T. Toward understanding monomeric ellagitannin biosynthesis. In Plant Polyphenols 2: Chemistry, Biology, Pharmacology, Ecology; Gross, G.G., Hemingway, R.W., Yoshida, T., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999; pp. 83–99. [Google Scholar]
- Davin, L.B.; Wang, H.-B.; Crowell, A.L.; Bedgar, D.L.; Martin, D.M.; Sarkanen, S.; Lewis, N.G. Stereoselective bimolecular phenoxy radical coupling by an auxiliary (dirigent) protein without an active center. Science 1997, 275, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Niehaus, J.U.; Gross, G.G. A gallotannin degrading esterase from leaves of Pedunculate oak. Phytochemistry 1997, 45, 1555–1560. [Google Scholar] [CrossRef]
- Hatano, T.; Yazaki, K.; Okonogi, A.; Okuda, T. Tannins of Stachyurus species II. praecoxin A, B, C and D, four new hydrolysable tannins from Stachyures praecox Leaves. Chem. Pharm. Bull. 1991, 39, 1689–1693. [Google Scholar] [CrossRef]
- Yoshida, T.; Nakata, F.; Hosotani, K.; Shingu, T.; Okuda, T. Tannins a Related Polyphenols of Melastomataceous Plants VII. Nobotanin J and K, trimeric and tetrameric hydrolysable Tannins from Heterocentron roseum. Chem. Pharm. Bull. 1995, 43, 1101–1106. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T.; Koga, T.; Toh, N.; Kurijama, K. Circular Discroism of hydrolyzable tannins II Dehydroellagitannins. Tetraedron Lett. 1982, 23, 3943–3944. [Google Scholar] [CrossRef]
- Regueiro, J.; Sánchez-González, C.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Lamuela-Raventós, R.; Izquierdo-Pulido, M. Comprehensive identification of walnut polyphenols by liquid chromatography coupled to linear ion trap–Orbitrap mass spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Saijo, R.; Nonaka, G.; Nishioka, I. Tannins and related compounds. LXXXIV. Isolation and characterization of five new hydrolyzable tannins from the bark of Mallotus japonicus. Chem. Pharm. Bull. 1989, 37, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Yoshida, T.; Ashida, M.; Yazaki, K. Tannins of Causarina and Stachyurus Species Part 1. Structures of Pedunculagin, Causarictin, Strictinin, Casuarinin, Casuariin and Stachyurin. J. Chem.Soc. Perkin Trans I 1983, 1765–1772. [Google Scholar] [CrossRef]
- Zhexiong, J.; Xin, L. Study on chemical constituents from leaves of Camellia. J. Chem. Pharm. Res. 2014, 6, 1770–1776. [Google Scholar]
- Isaza M., J.H. Polyphenolics of Colombian Melastomataceous plant Monochaetum multiflorum. Ph.D. Thesis, Okayama University, Okayama City, Japan, 2001. [Google Scholar]
- Hatano, T.; Yoshida, T.; Shingu, T.; Okuda, T. 13C Nuclear Magnetic Resonance Spectra of Hydrolyzable Tannins. II.: Tannins Forming Anomer Mixtures. Chem. Pharm. Bull. 1988, 36, 2925–2933. [Google Scholar] [CrossRef]
- Hatano, T.; Yoshida, T.; Shingu, T.; Okuda, T. 13C Nuclear Magnetic Resonance Spectra of Hydrolyzable Tannins. III. Tannins Having 1C4 Glucose and C-Glucosidic Linkage. Chem. Pharm. Bull. 1988, 36, 3849–3856. [Google Scholar] [CrossRef]
- Hatano, T.; Yazaki, K.; Okonoki, A.; Okuda, T. Tannins of Stachyurus Species II. Praecoxins A,B,C and D, four new hydrolyzable tannins from Stachyurus praecox leaves. Chem. Pharm. Bull. 1991, 39, 1689–1693. [Google Scholar]
- Okuda, T.; Yoshida, T.; Hatano, T. New Methods of Analyzing Tannins. J. Nat. Prod. 1989, 52, 1–31. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T.; Koga, T.; Toh, N.; Kuriyama, K. Circular dichroism of hydrolyzable tannins, I: Ellagitannins and gallotannins. Tetrahedron Lett. 1982, 23, 3937–3940. [Google Scholar] [CrossRef]
- Grace, M.H.; Warlick, C.W.; Neff, S.A.; Lila, M.A. Efficient preparative isolation and identification of walnut bioactive components using high-speed counter-current chromatography and LC-ESI-IT-TOF-MS. Food Chem. 2014, 158, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Yoshida, T.; Hatano, T. Classification of oligomeric hydrolyzables tannins and specifity of their ocurrence in plants. Phytochemistry 1993, 32, 507–521. [Google Scholar] [CrossRef]
- Jourdes, M.; Pouységu, L.; Deffieux, D.; Teissedre, P.-L.; Quideau, S. Hydrolyzable Tannins: Gallotannins and Ellagitannins. 2013. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T. Hydrolyzable tannins and related polyphenols. In Progress in the Chemistry of Organic Natural Products; Herz, W., Kirby, G.W., Moore, R.E., Steglich, W., Tamm, C., Eds.; Springer-Verlag: Vienna, Austria, 1995; Volume 66, pp. 1–117. [Google Scholar]
- Yoshida, T.; Ito, H.; Hipolito, I.J. Pentameric ellagitannin oligomers in melastomataceous plants—Chemotaxonomic significance. Phytochemistry 2005, 66, 1972–1983. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Haba, K.; Nakata, F.; Okano, Y.; Shingu, T. Tannins and Related polyphenols of Melastomataceous Plants III. Nobotanin G,H and I, Dimeric Hydrolyzable Tannins from Hetecentron roseum. Chem. Pharm. Bull. 1992, 40, 66–71. [Google Scholar] [CrossRef]
- Yoshida, T.; Nakata, F.; Nitta, A.; Okuda, T. Tannins and related polyphenols of Melastomataceous Plants V. Three new vomplex tannins from Melastoma malabathricum. Chem. Pharm. Bull. 1992, 40, 1727–1732. [Google Scholar] [CrossRef]
- Yoshida, T.; Nakata, F.; Okuda, T. Tannins and Related Polyphenols of Melastomataceous Plants VIII. Nobotanin L, M and N. Trimeric Hydrolyzable Tannins from Tibouchina semidecandra. Chem. Pharm. Bull. 1999, 47, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Amakura, Y.; Yokura, N.; Ito, H.; Isaza, J.H.; Ramirez, S.; Pelaez, D.P.; Renner, S.S. Oligomeric Hydrolyzable tannins from Tibouchina multiflora. Phytochemistry 1999, 52, 1661–1666. [Google Scholar] [CrossRef]
- Moura, V.M.; Sousa, L.A.F.; Oliveira, R.B.; Moura-Da-Silva, A.M.; Chalkidis, H.M.; Silva, M.N.; Pacheco, S.; Mourão, R.H.V. Inhibition of the principal enzymatic and biological effects of the crude venom of Bothrops atrox by plant extracts. J. Med. Plants Res. 2013, 7, 2330–2337. [Google Scholar]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serna, D.M.O.; Martínez, J.H.I. Phenolics and Polyphenolics from Melastomataceae Species. Molecules 2015, 20, 17818-17847. https://doi.org/10.3390/molecules201017818
Serna DMO, Martínez JHI. Phenolics and Polyphenolics from Melastomataceae Species. Molecules. 2015; 20(10):17818-17847. https://doi.org/10.3390/molecules201017818
Chicago/Turabian StyleSerna, Diana Marcela Ocampo, and José Hipólito Isaza Martínez. 2015. "Phenolics and Polyphenolics from Melastomataceae Species" Molecules 20, no. 10: 17818-17847. https://doi.org/10.3390/molecules201017818
APA StyleSerna, D. M. O., & Martínez, J. H. I. (2015). Phenolics and Polyphenolics from Melastomataceae Species. Molecules, 20(10), 17818-17847. https://doi.org/10.3390/molecules201017818