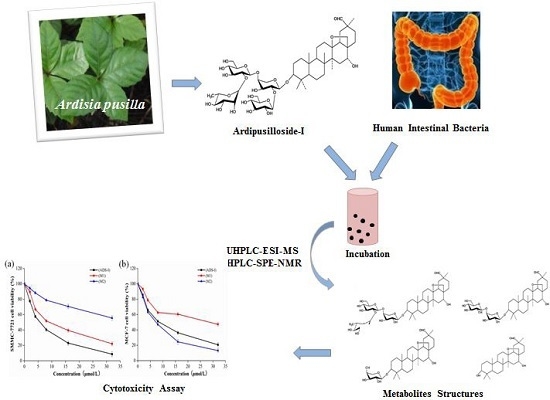
Ardipusilloside-I Metabolites from Human Intestinal Bacteria and Their Antitumor Activity
Abstract
:1. Introduction

2. Results
2.1. HPLC–ELSD Analysis

2.2. UHPLC–MS Analysis

| ADS-I and Its Metabolites | TR (min) | Possible Formula | Molecular Mass | [M − H]− (m/z) | MS2 Fragments |
|---|---|---|---|---|---|
| ADS-I | 2.4 | C53H86O22 | 1074 | 1073 | [1073]: 927, 765, 603 |
| [927]: 765, 603, 471 | |||||
| M1 | 4.3 | C47H76O17 | 912 | 911 | [911]: 765, 603, 471 |
| [765]: 603, 471 | |||||
| M2 | 5.1 | C41H66O13 | 766 | 765 | [765]: 603, 471 |
| M3 | 11.1 | C35H56O8 | 604 | 603 | [603]: 471, 407 |
| M4 | 22.0 | C30H48O4 | 472 | 471 | [471]: 423, 405, 377 |
| [423]: 405, 375 |
2.3. Sephadex LH-20 Chromatography
2.4. HPLC–SPE–NMR Analysis




2.5. Cytotoxicity Assay

3. Discussion

4. Experimental Section
4.1. Chemicals and Reagents
4.2. Preparation of Human Intestinal Bacterial Specimen
4.3. Metabolism of ADS-I by Human Intestinal Bacteria
4.4. HPLC–ELSD Analysis
4.5. UHPLC–MS Analysis
4.6. The Separation and Purification of ADS-I Metabolites
4.7. HPLC–SPE–NMR Analysis
4.8. Cell Culture
4.9. Cytotoxicity Assay
4.10. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Q.H.; Wang, X.J.; Miu, Z.C.; Feng, R. Chemical research on ardipusilloside. Acta Pharm. Sin. 1993, 28, 673–678. [Google Scholar]
- Liang, K.M.; Wang, S.W.; Wang, X. J. Inhibitory effect on of ardipusilloside on human cancer cell line. J. Forth Mil. Med. Univ. 2001, 22, 671–672. [Google Scholar]
- Dang, H.; Wang, J.; Cheng, J.X.; Wang, P.Y.; Wang, Y.; Cheng, L.F.; Caigan, Du.; Wang, X.J. Efficacy of local delivery of ardipusilloside I using biodegradable implants against cerebral tumor growth. Am. J. Cancer Res. 2015, 5, 243–254. [Google Scholar] [PubMed]
- Wang, S.W.; Xie, Y.H.; Liu, J.; Wang, X.J. Internal anticancer action of ardipusilloside on rats. J. Forth Mil. Med. Univ. 2000, 21, 236–237. [Google Scholar]
- Tao, X.J.; Wang, P.X.; Yang, X.J.; Yao, H.P.; Liu, J.; Cao, Y.S. Inhibitory effect of Ardipusilloside-I on Lewis pulmonary carcinoma and hepatocarcinoma SMMC-7721. J. Chin. Med. Mater. 2005, 28, 574–577. [Google Scholar]
- Tao, X.J.; Long, J.W.; He, J.Y.; Yao, H.P.; Liu, J.; Cao, Y.S. The anti-tumor and immune regulation effect of Ardipusilloside-I. Chin. Phamacol. Bull. 2005, 21, 1070–1073. [Google Scholar]
- Li, M.; Li, W.F.; Qin, L.; Zhou, J. Internal anticancer action of the saponins from Ardisia pusilla DC. J. Guangxi Tradit. Chin. Med. Univ. 2004, 7, 11–12. [Google Scholar]
- Wang, X.J.; Cui, H.; Wang, R.; Huan, ML.; Zhang, B.L.; Zhang, W.D.; Teng, Z.H.; Gan, H.Q.; Zhou, S.Y.; Gu, Y. Metabolism and pharmacokinetic study of Ardipusilloside I in rats. Planta Med. 2012, 78, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.A.; Choo, M.K.; Park, E.K.; Park, S.K.; Shin, H.Y.; Kim, D.H. Metabolism of ginsenoside R(c) by human intestinal bacteria and its related antiallergic activity. Biol. Pharm. Bull. 2002, 25, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.A.; Han, M.J.; Choo, M.K.; Kim, DH. Metabolism of 20(S)-and 20(R)-ginsenoside Rg3 by human intestinal bacteria and its relation to in vitro biological activities. Biol. Pharm. Bull. 2002, 25, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Jhonny, C.R.; Diana, M.; Guillermo, M.; Diang, M.; Maria-Elena, M.; Juan, L.; Alejandro, M. HPLC-ESI-IT-MS/MS analysis and biological activity of triterpene glycosides from the Colombian marine sponge Ectyoplasia ferox. Mar. Drugs 2013, 11, 4815–4833. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.-H.; Peng, Y.; Wang, M.-Y.; Chen, D.-F.; Li, X-B. In vitro human fecal microbial metabolism of Forsythoside A and biological activities of its metabolites. Fitoterapia 2014, 99, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.Y.; Liu, P.; Wang, H.Y.; Qi, L.W.; Wang, C.Z.; Li, P.; Yuan, C.S. Biotransformation and metabolic profile of American ginseng saponins with human intestinal mtcroflora by liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2013, 86, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.A.; Shin, J.E.; Kim, D.H. Metabolism of Ginsenoside Re by human intestinal microflora and its estrogenic effect. Biol. Pharm. Bull. 2005, 29, 1903–1908. [Google Scholar] [CrossRef]
- Yan, L.; Yang, X.-L.; Meng, Z.-Q.; Yuan, Y.; Xiao, W.; Wang, Z.; Huang, W.; Yang, Z.; Zhang, C. Simultaneous quantification of Akebia saponin D and its five metabolites in human intestinal bacteria using ultra-performance liquid chromatography triple quadrupole mass spectrometry. J. Chromatogr. B 2014, 971, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Ha, Y.W.; Na, Y.; Ha, I.J.; Kim, D.H.; Kim, Y.S. Liquid chromatography/mass spectrometry-based structural analysis of new platycoside metabolites transformed by human intestinal bacteria. J. Pharm. Biomed. Anal. 2010, 51, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.W.; Cao, J.; Li, P.; Yu, Q.T.; Wen, X.D.; Wang, Y.X.; Li, C.Y.; Bao, K.D.; Ge, X.X.; Cheng, X.L. Qualitative and quantitative analysis of Radix astragali products by fast high-performance liquid chromatography-diode array detection coupled with time-of-flight mass spectrometry through dynamic adjustment of fragmentor voltage. J. Chromatogr. A 2008, 3, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yuan, Q.; Liu, E.H.; Qi, L.W.; Bi, Z.M.; Li, P. Fragmentation study of iridoid glycosides and phenylpropanoid glycosides in Radix scrophulariae by rapid resolution liquid chromatography with diode-array detection and electrospray ionization time-of-flight mass spectrometry. Biomed. Chromatogr. 2010, 24, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhu, Z.Y.; Yang, L.; Gao, Y.; Liu, W.; Zhang, H.; Chai, Y. Detection, characterization and identification of major constituents in Zhimu-Baihe herb-pair extract by fast high-performance liquid chromatography and time-of-flight mass spectrometry through dynamic adjustment of fragmentor voltage. Rapid Commun. Mass Spectrom. 2011, 25, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Uchiyama, M. Antimetastatic efficacy of orally administered ginsenoside Rb1 in dependence on intestinal bacterial hydrolyzing potential and significance of treatment with an active bacterial metabolite. Planta Med. 1998, 64, 696–700. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Sung, J.-H.; Lee, S.-J.; Moon, C.-K.; Lee, B.-H. Antitumor activity of a novel ginseng saponin metabolite in human pulmonary adenocarcinoma cells resistant to cisplatin. Cancer Lett. 1999, 144, 39–43. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.J.; Sun, X.L. Separation and purification of Ardipusilloside I by high performance liquid chromatography. Chin. Tradit. Herb. Drugs 2008, 39, 1178–1180. [Google Scholar]
- Chang, S.-Y.; Han, M.J.; Joh, E.-H.; Kim, DH. Liquid chromatography/mass spectrometry-base structural analysis of soyasaponin Ab metabolites by human fecal microflora. J. Pharm. Biomed. Anal. 2010, 52, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.-Q.; Leong, W.-I.; Yan, R.; Wang, Y-T. Characterization of Metabolism and in vitro Permeability study of Notoginsenoside R1 from Radix notoginseng. J. Agric. Food Chem. 2010, 58, 5770–5776. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, W.-Y.; Wang, Y.-N.; Wang, P.-Y.; Lei, W.; Feng, B.; Wang, X.-J. Ardipusilloside-I Metabolites from Human Intestinal Bacteria and Their Antitumor Activity. Molecules 2015, 20, 20569-20581. https://doi.org/10.3390/molecules201119719
Cao W-Y, Wang Y-N, Wang P-Y, Lei W, Feng B, Wang X-J. Ardipusilloside-I Metabolites from Human Intestinal Bacteria and Their Antitumor Activity. Molecules. 2015; 20(11):20569-20581. https://doi.org/10.3390/molecules201119719
Chicago/Turabian StyleCao, Wei-Yu, Ya-Nan Wang, Peng-Yuan Wang, Wan Lei, Bin Feng, and Xiao-Juan Wang. 2015. "Ardipusilloside-I Metabolites from Human Intestinal Bacteria and Their Antitumor Activity" Molecules 20, no. 11: 20569-20581. https://doi.org/10.3390/molecules201119719
APA StyleCao, W. -Y., Wang, Y. -N., Wang, P. -Y., Lei, W., Feng, B., & Wang, X. -J. (2015). Ardipusilloside-I Metabolites from Human Intestinal Bacteria and Their Antitumor Activity. Molecules, 20(11), 20569-20581. https://doi.org/10.3390/molecules201119719