A Nanostructured Lipid System as a Strategy to Improve the in Vitro Antibacterial Activity of Copper(II) Complexes
Abstract
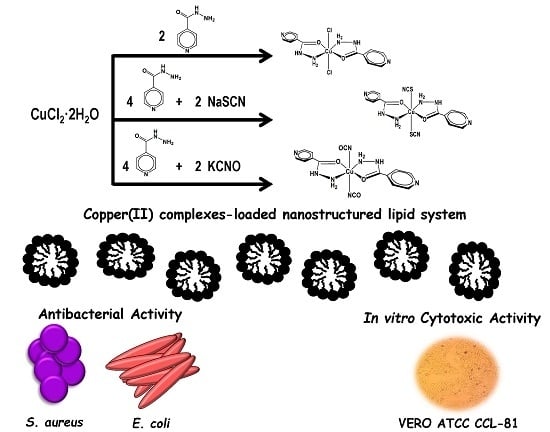
:1. Introduction




2. Results and Discussion
2.1. Chemistry
2.1.1. Infrared (IR) Spectroscopy Studies
| Compound | Assignment | |||
|---|---|---|---|---|
| INH | 1 | 2 | 3 | |
| - | 3437 m | 3434 m | 3449 w | νOH |
| 3306 m, 3213 sh | 3222 s | 3241 s | 3236 w | νasNH2 |
| 3112 s | 3098 s | 3101 sh | 3108 w | νsiNH2 |
| 3035 m, 3014 m | 3048 s, 3019 m | 3057 sh | 3061 sh, 3017 w | νCH |
| - | - | - | 2212 s | νasNCO |
| - | - | 2130 s | - | νasNCS |
| 1667 s | 1653 m | 1606 w | 1615 m | νC=O |
| 1635 m | 1648 s | 1648 m | 1655 sh | Scissoring NH2 |
| 1603 m | 1590 s | 1603 s | 1589 sh | νring + δCH +δHOH |
| 1556 s | 1547 s | 1554 m | 1537 m | νring |
| 1492 w | 1494 w | 1498 w | 1499 w | δCH + νring + δNH |
| 1412 m | 1415 m | 1415 m | 1416 m | δCH + νring |
| - | - | - | 1317 w | νsNCO |
| 1335 s | 1332 m | - | - | Rocking NH2 |
| - | 1284 w | 1268 w | 1283 w | νring |
| 1221 m | 1225 m, 1202 s | 1233 w, 1208 w | 1239 w | δCH + νring |
| 1141 m | 1139 m | 1131 w, 1098 w | 1131 w, 1103 w | δCH + νring + νCN |
| 1060 s | 1065 w | 1058 m | 1058 w | νring + δCH + δring |
| 996 m | 996 w | 988 w | 987 sh | νring + δring |
| 943 s | - | 941 w, 919 w | 945 w, 924 w | νNN + twisting NH2 + νNH2 |
| 888 w, 845 m | 906 w, 881 w, 858 m | 876 w | 868 sh | γCH + γC-CO-NH-NH2 |
| 747 w | 761 w | 755 w | 775 w, 756 w | γCH + τring + γCO +ν NC-S |
| - | 712 m | 715 m | 719 w | τring + γCO + γCH |
| 675 s | - | 690 m | 692 w | δring + νC-CO-NH-NH2 |
| 659 s | - | 664 sh | 658 w | δring + δCH |
| - | - | - | 620 w | δNCO |
| 504 w | 538 w | 504 w | 501 sh | τHNNH + γNH + δNCC + δC-CO-NH-NH2 + δOCC |
| 436 w | 463 w | 466 w, 430 w | 420 w | γNH + τHNNH + γCH + τring + δNCS |
2.1.2. Ultraviolet-Visible (UV-Vis) Spectroscopy Studies
| Compound | IL Transition | LMCT Transition | LF Transition | Ref. |
|---|---|---|---|---|
| INH | 249 (3120) | - | - | This work |
| [CuCl2(INH)2]·H2O (1) | 259 (2930) | 352 (1170) | 678 (228) | This work |
| [Cu(NCS)2(INH)2]·5H2O (2) | 249 (3000) | 353 (1000) | 688 (184) | This work |
| [Cu(NCO)2(INH)2]·4H2O (3) | 273 (1440) | 356 (1120) | 678 (233) | This work |
2.1.3. Electronic Paramagnetic Resonance (EPR) Studies and Proposed Structures for the New Complexes

2.2. Nanostructured Lipid System
| Formulation | Mean Diameter ± S.D. (nm) * | Mean PDI ± S.D. * |
|---|---|---|
| ME | 125.6 ± 1.804 | 0.246 ± 0.008 |
| 1-Loaded | 158.0 ± 1.060 | 0.228 ± 0.011 |
| 2-Loaded | 212.6 ± 1.539 | 0.284 ± 0.034 |
| 3-Loaded | 171.7 ± 1.947 | 0.218 ± 0.007 |
2.3. In Vitro Biological Activity
| Complexes | Parameters | |||||
|---|---|---|---|---|---|---|
| S. aureus | E. coli | |||||
| MIC (μg/mL) | IC50 (μg/mL) | SI | MIC (μg/mL) | IC50 (μg/mL) | SI | |
| 1-Not loaded | 1000 | 109.5 | 0.1095 | 2000 | 109.5 | 0.05475 |
| 1-Loaded | 250 | 319.3 | 1.288 | 125 | 319.3 | 2.554 |
| 2-Not loaded | 500 | 314.3 | 0.6286 | 2000 | 314.3 | 0.1571 |
| 2-Loaded | 500 | 329.3 | 0.6586 | 125 | 329.3 | 2.634 |
| 3-Not loaded | 1000 | 325.3 | 0.3253 | 8000 | 325.3 | 0.040 |
| 3-Loaded | 125 | >500 | 4.000 | 500 | >500 | 1.000 |
3. Experimental Section
3.1. General Information
3.2. Synthesis of the Copper(II) Complexes
3.2.1. [CuCl2(INH)2]·H2O (1)
3.2.2. [Cu(NCS)2(INH)2]·5H2O (2)
3.2.3. [Cu(NCO)2(INH)2]·4H2O (3)
3.3. Characterization of the Copper(II) Complexes
3.3.1. IR Spectroscopy
3.3.2. UV-Vis Spectroscopy
3.3.3. EPR Spectroscopy
3.3.4. Melting Point Determination
3.3.5. Elemental Analysis
3.3.6. Complexometry with 2,2′,2′′,2′′′-(Ethane-1,2-diyldinitrilo)tetraacetic Acid (EDTA)
3.4. Nanostructured Lipid System Preparation
3.5. Nanostructured Lipid System Characterization: Mean Diameter and Polydispersity Index (PDI)
3.6. Preparation of the Coordination Compound-Loaded Nanostructured Lipid System
3.7. Antibacterial Activity
3.8. In Vitro Cytotoxic Activity
3.9. Selectivity Index (SI)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bibi, Y.; Nisa, S.; Chaudhary, F.M.; Zia, M. Antibacterial activity of some selected medicinal plants of Pakistan. BMC Complement. Altern. Med. 2011, 11, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Nikaido, H. Multidrug resistance in bacteria. Annu. Rev. Biochem. 2009, 78, 119–146. [Google Scholar] [CrossRef] [PubMed]
- Sorenson, J., Jr. Copper complexes offer a physiological approach to treatment of chronic diseases. Prog. Med. Chem. 1989, 26, 437–568. [Google Scholar] [PubMed]
- Weder, J.E.; Dillon, C.T.; Hambley, T.W.; Kennedy, B.J.; Lay, P.A.; Biffin, J.R.; Regtop, H.L.; Davies, N.M. Copper complexes of non-steroidal anti-inflammatory drugs: An opportunity yet to be realized. Coord. Chem. Rev. 2002, 323, 95–126. [Google Scholar] [CrossRef]
- Sato, M.R.; Silva, P.B.; Souza, R.A.; Santos, K.C.; Chorilli, M. Recent advances in nanoparticle carriers for coordination complexes. Curr. Top. Med. Chem. 2015, 15, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.N.; Joshi, H.N.; Patel, C.R. Copper(II) complexes with norfloxacin and neutral terpyridines: cytotoxic, antibacterial, superoxide dismutase and DNA-interaction approach. Polyhedron 2012, 40, 159–167. [Google Scholar] [CrossRef]
- Xu, D.F.; Shen, Z.H.; Shi, Y.; He, Q.; Xia, Q.C. Synthesis, characterization, crystal structure, and biological activity of the copper complex. Russ. J. Coord. Chem. 2010, 36, 458–462. [Google Scholar] [CrossRef]
- Pahontu, E.; Ilies, D.-C.; Shova, S.; Paraschivescu, C.; Badea, M.; Gulea, A.; Rosu, T. Synthesis, characteriation, crystal structure and antimicrobial activity of copper(II) complexes with the schiff base derived from 2-hydroxy-4-methoxybenzaldehyde. Molecules 2015, 20, 5771–5792. [Google Scholar] [CrossRef] [PubMed]
- Freitas, E.S.; Silva, P.B.; Chorilli, M.; Batista, A.A.; Lopes, E.O.; Silva, M.M.; Leite, C.Q.F.; Pavan, F.R. Nanostructured lipid systems as a strategy to improve the in vitro cytotoxicity of ruthenium(II) compounds. Molecules 2014, 19, 5999–6008. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.B.; Freitas, E.S.; Bernegossi, J.; Gonçalez, M.L.; Sato, M.R.; Leite, C.Q.F.; Pavan, F.R.; Chorilli, M. Nanotechnology-based drug delivery systems for treatment of tuberculosis—A review. J. Biomed. Nanotech. 2016, 12, 241–260. [Google Scholar]
- Santos, F.K.; Oyafuso, M.H.; Kiill, C.P.; Gremião, M.P.D.; Chorilli, M. Nanotechnology-based drug delivery systems for treatment of hyperproliferative skin diseases—A review. Curr. Nanosci. 2013, 9, 159–167. [Google Scholar]
- Souza, A.L.R.; Kiill, C.P.; Santos, F.K.; Luz, G.M.; Chorilli, M.; Gremião, M.P.D. Nanotechnology-based drug delivery systems for dermatomycosis treatment. Curr. Nanosci. 2012, 8, 512–519. [Google Scholar] [CrossRef]
- Yadav, H.D.S.; Sengupta, S.K.; Tripathi, S.C. Oxozirconium(IV) complexes of terephthalic acid dihydrazide and their reactions with β-diketones. Synth. React. Inorg. Met. Org. Chem. 1987, 17, 115–127. [Google Scholar] [CrossRef]
- Donia, A.M.; El-Rayes, M. Thermochromism of the cobalt(II)-isoniazid complex in the solid state. Thermochim. Acta 1989, 147, 65–70. [Google Scholar] [CrossRef]
- Akyuz, S. The FT-IR spectra of pyrazinamide complexes of transition metal(II) tetracyanonickelate. J. Mol. Struct. 2003, 651–653, 541–545. [Google Scholar] [CrossRef]
- Nakamnot, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Lucca Neto, V.A.; Mauro, A.E.; Ananias, S.R.; Stevanato, A.; Moro, A.C. Synthesis and the use of [Pd(dmba)(NCO)(PPh3)] in catalytic processes involving the preparation of urea derivatives and alkoxycarbonyl complexes. Eclet. Quim. 2006, 31, 75–81. [Google Scholar]
- Miskowski, V.M.; Thich, J.A.; Solomon, R.; Schugar, H.J. Electronic spectra of substituted copper(II) thioether complexes. J. Am. Chem. Soc. 1976, 98, 8344–8350. [Google Scholar] [CrossRef]
- Dockum, B.W.; Reiff, W.M. Thyocyanate-bridged transition-metal polymers. 1. Structure and low-temperature magnetic behavior of (bipyridyl)iron(II) thiocyanate: Fe(2,2′-(bpy)(NCS)2. Inorg. Chem. 1982, 21, 391–398. [Google Scholar] [CrossRef]
- Sargentelli, V. Synthesis, Study of Thermal and Eletrochemical Behavior and Reactivity of Pseudohalides Copper(II) Complexes. Ph.D. Thesis, Chemistry Institute—Universidade Estadual Paulista, Araraquara, Brazil, 1986. [Google Scholar]
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier: New York, NY, USA, 1986; p. 420. [Google Scholar]
- Van Ooijen, J.A.C.; van der Put, P.J.; Reedijk, J. Compressed tetragonal geometry in Cu(II) doped dichlorobis(pyrazole)cadmium. Chem. Phys. Lett. 1977, 51, 380–382. [Google Scholar] [CrossRef]
- Chandra, S.; Gupta, L.K. EPR and electronic spectral studies on Co(II), Ni(II) and Cu(II) complexes with a new tetradentate [N4] macrocyclic ligand and their biological activity. Spectrochim. Acta A 2004, 60, 1563–1571. [Google Scholar] [CrossRef] [PubMed]
- Goyal, U.; Arora, R.; Aggarwal, G. Formulation design and evaluation of a self-microemulsifying drug delivery system of lovastatin. Acta Pharm. 2012, 62, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Cunha Junior, A.; Fialho, S.L.; Carneiro, L.B.; Oréfice, F. Microemulsions as drug delivery systems for topical ocular administration. Arq. Bras. Oftalmol. 2003, 63, 285–391. [Google Scholar]
- Afanaséva, G.V.; Bychkova, T.I.; Shtyrlin, V.G.; Shakirova, A.R.; Garipov, R.R.; Zyavkina, Y.I.; Zakharov, A.V. Complexation and ligand exchange in aqueous of Cu(II) and Ni(II) with hydrazides of some aromatic acids. Russ. J. Gen. Chem. 2006, 76, 346–355. [Google Scholar]
- Oliveira, C.N.; Ionashiro, M.; Graner, C.A.F. Complexometric titration of zinc, copper and cobalt. Eclet. Quim. 1985, 10, 7–10. [Google Scholar]
- Bonifácio, B.V.; Ramos, M.A.S.; Silva, P.B.; Negri, K.M.S.; Lopes, E.O.; Souza, L.P.; Vilegas, W.; Pavan, F.R.; Chorilli, M.; Bauab, T.M. Nanostructured lipid system as a strategy to improve the anti-Candida albicans activity of Astronium sp. Int. J. Nanomed. 2015, 10, 5081–5092. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. In Approved Standard, 6th ed.; Document M7-A6 Performance Standards for Antimicrobial Susceptibility Testing; CLSI: Wayne, PA, USA, 2006. [Google Scholar]
- Pavan, F.R.; Maia, P.I.; Leite, S.R.A.; Deflon, V.M.; Batista, A.A.; Sato, D.N.; Franzblau, S.G.; Leite, C.Q.F. Thiosemicarbazones, semicarbazones, dithiocarbazates and hydrazide/hydrazones: Anti-Mycobacterium tuberculosis activity and cytotoxicity. Eur. J. Med. Chem. 2010, 45, 1898–1905. [Google Scholar] [CrossRef] [PubMed]
- Valdivieso-Garcia, A.; Clarke, R.C.; Rahn, K.; Durette, A.; MacLeod, D.L.; Gyles, C.L. Neutral red assay for measurement of quantitative vero cell cytotoxicity. Appl. Environ. Microbiol. 1993, 59, 1981–1983. [Google Scholar] [PubMed]
- Orme, I. Search for new drugs for treatment of tuberculosis. Antimicrob. Agents Chemother. 2001, 45, 1943–1946. [Google Scholar] [PubMed]
- Sample Availability: Samples of the compounds CuCl2(INH)2]·H2O, [Cu(NCS)2(INH)2]·5H2O and [Cu(NCO)2(INH)2]·4H2O are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Silva, P.B.; Bonifácio, B.V.; Frem, R.C.G.; Godoy Netto, A.V.; Mauro, A.E.; Ferreira, A.M.d.C.; Lopes, E.D.O.; Raddi, M.S.G.; Bauab, T.M.; Pavan, F.R.; et al. A Nanostructured Lipid System as a Strategy to Improve the in Vitro Antibacterial Activity of Copper(II) Complexes. Molecules 2015, 20, 22534-22545. https://doi.org/10.3390/molecules201219822
Da Silva PB, Bonifácio BV, Frem RCG, Godoy Netto AV, Mauro AE, Ferreira AMdC, Lopes EDO, Raddi MSG, Bauab TM, Pavan FR, et al. A Nanostructured Lipid System as a Strategy to Improve the in Vitro Antibacterial Activity of Copper(II) Complexes. Molecules. 2015; 20(12):22534-22545. https://doi.org/10.3390/molecules201219822
Chicago/Turabian StyleDa Silva, Patricia B., Bruna V. Bonifácio, Regina C. G. Frem, Adelino V. Godoy Netto, Antonio E. Mauro, Ana M. da Costa Ferreira, Erica De O. Lopes, Maria S. G. Raddi, Tais M. Bauab, Fernando R. Pavan, and et al. 2015. "A Nanostructured Lipid System as a Strategy to Improve the in Vitro Antibacterial Activity of Copper(II) Complexes" Molecules 20, no. 12: 22534-22545. https://doi.org/10.3390/molecules201219822
APA StyleDa Silva, P. B., Bonifácio, B. V., Frem, R. C. G., Godoy Netto, A. V., Mauro, A. E., Ferreira, A. M. d. C., Lopes, E. D. O., Raddi, M. S. G., Bauab, T. M., Pavan, F. R., & Chorilli, M. (2015). A Nanostructured Lipid System as a Strategy to Improve the in Vitro Antibacterial Activity of Copper(II) Complexes. Molecules, 20(12), 22534-22545. https://doi.org/10.3390/molecules201219822