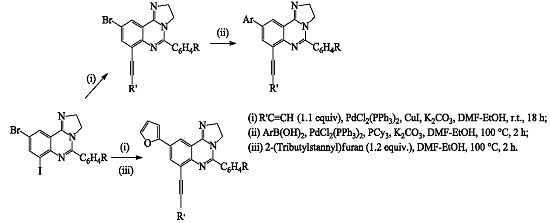
3. Experimental Section
3.1. General Information
Melting points were recorded on a Thermocouple digital melting point apparatus and are uncorrected. IR spectra were recorded as powders using a Bruker VERTEX 70 FT-IR Spectrometer (Bruker Optics, Billerica, MA, USA) with a diamond ATR (attenuated total reflectance) accessory by using the thin-film method. For column chromatography, Merck Kieselgel 60 (0.063–0.200 mm) (Merck KGaA, Frankfurt, Germany) was used as stationary phase. NMR spectra were obtained as CDCl
3 or DMSO-
d6 solutions using an Agilent 500 MHz NMR spectrometer (Agilent Technologies, Oxford, UK) and the chemical shifts are quoted relative to the TMS peak. Low- and high-resolution mass spectra were recorded at an ionization potential of 70 eV using Synapt G2 Quadrupole Time-of-flight mass spectrometer (Waters Corp., Milford, MA, USA) at the University of Stellenbosch Mass Spectrometry Unit. The synthesis and characterization of compounds
1a–
c has been described before [
10].
3.2. Typical Procedure for Preparation of the 2-(3-Aryl-6-bromo-8-iodoquinazolin-4-yl)ethanols 2a–c
A stirred mixture of
1a (1.00 g, 2.24 mmol) and 3-aminoethanol (20 mL) was heated under reflux for 2 h. The mixture was allowed to cool to room temperature and then quenched slowly with an ice-cold water. The resulting precipitate was filtered and recrystallized from to afford
2. The following products were prepared in this fashion: (The
1H- and
13C-NMR spectra of compounds
2–
6 are listed in the
Supplementary Materials).
2-(6-Bromo-8-iodo-2-phenylquinazolin-4-yl)amino)ethanol (2a). Solid (0.95 g, 90%), mp. 256–257 °C (toluene); νmax (ATR) 743, 796, 846, 956, 1013, 1080, 1349, 1455, 1518, 1549, 1590, 3316 cm−1; 1H-NMR (500 MHz, DMSO-d6) δH 3.32 (1H, s, OH), 3.72 (2H, t, J = 5.5 Hz, CH2N-), 3.75 (2H, t, J = 5.5 Hz, -CH2O-), 4,82 (1H, t, J = 5.5 Hz, -NH), 7.52–7.54 (3H, m, 3′,4′,5′-H), 8.45 (1H, d, J = 1.5 Hz, 5-H), 8.53–8.58 (2H, m, 2′,6′-H), 8.61 (1H, d, J = 1.5 Hz, 8-H); 13C-NMR δC (125 MHz, DMSO-d6) 44.5, 59.4, 104.8, 115.5, 118.2, 126.3, 128.6, 128.8, 131.1, 138.3, 144.4, 148.7, 160.0, 160.8; MS m/z 470 (100, MH+); HRMS (ES): MH+, found 469.9356. C16H14N3O79BrI+ requires 469.9365.
2-((6-Bromo-2-(4-fluorophenyl)-8-iodoquinazolin-4-yl)amino)ethanol (2b). Solid (0.90 g, 85%), mp. 252–253 °C (toluene); νmax (ATR) 706, 797, 859, 959, 1054, 1081, 1146, 1220, 1347, 1421, 1457, 1509, 1549, 1588, 3299 cm−1; δH (500 MHz, DMSO-d6) 3.31 (1H, s, -OH), 3.71 (2H, t, J = 5.5 Hz, -CH2N-), 3.74 (2H, t, J = 5.5 Hz, -CH2O-), 4,83 (1H, t, J = 5.5 Hz, -NH), 7.36 (2H, t, J = 9.0 Hz, 3′,5′-H), 8.45 (1H, d, J = 2.0 Hz, 5-H), 8.57 (2H, t, J = 9.0 Hz, 2′,6′-H), 8.60 (1H, d, J = 2.0 Hz, 7-H); 13C-NMR δC (125 MHz, DMSO-d6) 44.4, 59.4, 104.6, 115.4, 115.7 (d, 1JCF = 21.8 Hz), 118.2, 126.3, 130.8 (d, 3JCF = 8.6 Hz), 138.3 (d, 4JCF = 2.9 Hz), 144.5, 148.7, 159.9, 160.0, 164.4 (d, 2JCF 246.5 Hz); MS m/z 488 (100, MH+); HRMS (ES): MH+, found 488.9269. C16H13N3O79BrFI+ requires 488.9271.
2-((6-Bromo-2-(4-chlorophenyl)-8-iodoquinazolin-4-yl)amino)ethanol (2c). Solid (0.92 g, 87%), mp. 260–261 °C (toluene); νmax (ATR) 797, 847, 859, 957, 1014, 1054, 1089, 1350, 1420, 1519, 1559, 1591, 3325 cm−1; δH (500 MHz, DMSO-d6) 3.31 (1H, s, -OH), 3,71 (2H, t, J = 5.5 Hz, -CH2N-), 3.71 (2H, t, J = 5.5 Hz, -CH2O-), 4,83 (1H, t, J = 5.5 Hz, -NH), 7.61 (2H, d, J = 8.5 Hz, 3′,5′-H), 8.46 (1H, d, J = 2.0 Hz, 5-H), 8.52 (2H, d, J = 8.5 Hz, 2′,6′-H), 8.62 (1H, d, J = 2.0 Hz, 7-H); 13C-NMR δC (125 MHz, DMSO-d6) 46.5, 59.4, 104.7, 115.5, 118.4, 126.3, 128.9, 130.2, 136.0, 137.2, 144.5, 148.6, 159.8, 160.0; MS m/z 504 (100, MH+); HRMS (ES): MH+, found 503.8979. C16H13N3O35Cl79BrI+ requires 503.8975.
3.3. Typical Procedure for Preparation of the 5-Aryl-9-bromo-7-iodo-2,3-dihydroimidazo[1,2-c]quinazolines 3a–c
9-Bromo-7-iodo-5-phenyl-2,3-dihydroimidazo[1,2-c]quinazoline (3a). A stirred mixture of 2a (1.00 g, 2.13 mmol) and H2SO4 (30 mL) was heated at 120 °C for 2 h. The mixture was allowed to cool to room temperature and then added slowly to an ice-cold water (100 mL). The pH of the dilute acidic mixture was adjusted to 8–10 with 25% aqueous NaOH solution with stirring. The resultant precipitate was filtered and recrystallized to afford 3a as a solid (0.87 g, 90%), mp. 241–242 °C (toluene); νmax (ATR) 568, 692, 773, 879, 1004, 1312, 1441, 1581, 1659 cm−1; 1H-NMR δH (500 MHz, DMSO-d6) 3.40 (2H, t, J = 9.0 Hz, -CH2N-), 4,18 (2H, t, J = 9.0 Hz, -CH2N=), 7.53–7.61 (3H, m, 3′,4′,5′-H), 7.79 (2H, d, J = 8.5 Hz, 2′,6′-H), 8.05 (1H, d, J = 2.0 Hz, 5H), 8.35 (1H, d, J = 2.0 Hz, 7-H); 13C-NMR δC (125 MHz, DMSO-d6) 44.3, 47.7, 118.7, 123.0, 125.7, 126.8, 128.6, 128.9, 130.3, 135.3, 137.3, 141.5, 143.8, 157.5; MS m/z 452 (100, MH+); HRMS (ES): MH+, found 451.9359. C16H12N379BrI+ requires 451.9359.
9-Bromo-5-(4-fluorophenyl)-7-iodo-2,3-dihydroimidazo[1,2-c]quinazoline (3b). Solid (0.85 g, 88%), mp. 253–254 °C (toluene); νmax (ATR) 580, 705, 783, 860, 1228, 1356, 1405, 1563, 1649 cm−1; 1H-NMR δH (500 MHz, DMSO-d6) 4.02 (2H, t, J = 9.5 Hz, -CH2N-), 4.25 (2H, t, J = 9.5 Hz, CH2N=), 7.41 (2H, t, J = 8.5 Hz, 3′,5′-H), 7,88 (2H, t, J = 8.5 Hz, 2′,6′-H), 8.11 (1H, d, J = 2.0 Hz, 5-H), 8.40 (1H, d, J = 2.0 Hz, 7-H); 13C-NMR δC (125 MHz, DMSO-d6) 49.4, 52.2, 102.8, 116.1 (d, 1JCF = 21.8 Hz), 117.9, 119.5, 127.8, 130.7 (d, 4JCF = 2.6 Hz), 131.5 (d, 3JCF = 8.6 Hz), 145.8, 146.4, 154.5, 162.9, 164.9 (d, 1JCF = 247.5 Hz); MS m/z 470 (100, MH+); HRMS (ES): MH+, found 469.9161. C16H11N379 BrIF+ requires 469.9165.
9-Bromo-5-(4-chlorophenyl)-7-iodo-2,3-dihydroimidazo[1,2-c]quinazoline (3c). Solid (0.88 g, 92%), mp. 224–225 °C (toluene); νmax (ATR) 620, 782, 996, 1065, 1092, 1301, 1357, 1556, 1577, 1652 cm−1; 1H-NMR δH (500 MHz, DMSO-d6) 3.71 (2H, t, J = 9.5 Hz, CH2N-), 3.74 (2H, t, J = 9.5 Hz, CH2N=), 7.60 (2H, d, J = 8.5 Hz, 3′,5′-H), 8.46 (1H, d, J = 2.0 Hz, 5-H), 8.52 (2H, d, J = 8.5 Hz, 2′,6′-H), 8.62 (1H, d, J = 2.0 Hz, 7-H); 13C-NMR δC (125 MHz, DMSO-d6) 49.0, 54.4, 102.5, 118.9, 119.6, 127.6, 128.9, 130.6, 133.5, 135.9, 144.5, 146.2, 153.2, 154.3; MS m/z 485 (100, MH+); HRMS (ES): MH+, found 485.8881. C16H11N335Cl79BrI+ requires 485.8670.
3.4. Typical Procedure for the Site-Selective Sonogashira Cross-Coupling of 3a–c with Terminal Alkynes
9-Bromo-5-phenyl-7-(phenylethynyl)-2,3-dihydroimidazo[1.2-c]quinazoline (4a). A stirred mixture of 3a (0.50 g, 1.106 mmol), PdCl2 (PPh3)2 (0.04 g, 0.06 mmol), CuI (0.02 g; 0.11 mmol) and K2CO3 (0.23 g, 1.66 mmol) in 3:1 DMF–EtOH (v/v, 15 mL) was purged with argon gas for 30 min. Phenylacetylene (0.12 g, 1.22 mmol) was added to the mixture using a syringe. The reaction mixture was stirred at room temperature for 18 h and then quenched with ice-cold water. The product was extracted into chloroform and the combined organic layers were washed with water, dried over Na2SO4, filtered and evaporated under reduced pressure. The residue was purified by column chromatography on silica gel to afford 4a as a yellow solid (0.31 g, 66%), Rf (ethyl acetate) 0.47, mp. 212‒213 °C; νmax (ATR) cm−1 691, 753, 1015, 1090, 1283, 1455, 1538, 1642, 2870, 2923 cm−1; 1H-NMR δH (500 MHz, CDCl3) 4.01–4.18 (4H, m, -CH2CH2-), 7.31–7.34 (3H, m, 3′,4′,5′-H), 7.49–7.54 (5H, m, Ph), 7.76–7.78 (2H, m, 2′,6′-H), 7.88 (1H, d, J = 2.0 Hz, 8-H), 8.14 (1H, d, J = 2.0 Hz, 10-H); 13C-NMR δC (125 MHz, CDCl3) 49.2, 53.8, 85.6, 96.5, 118.9, 119.5, 123.0, 123.4, 127.8, 128.3, 128.8, 129.8, 131.8, 133.0, 136.9, 139.2, 146.3, 153.0, 154.4; 426; MS m/z (100, MH+); HRMS (ES): MH+ found 426.0604. C24H17N379Br+ requires 426.0606.
9-Bromo-5-(4-fluorophenyl)-7-(phenylethynyl)-2,3-dihydroimidazo[1.2-c]quinazoline (4b). Solid (0.30 g, 64%), Rf (ethyl acetate) 0.5, mp. 194–195 °C; νmax (ATR) 508, 559, 693, 713, 756, 836, 1158, 1237, 1377, 1423, 1510, 1604, 1642, 2870, 2922 cm−1; 1H-NMR δH (500 MHz, CDCl3) 4.10–4.16 (4H, m, -CH2CH2-), 7.19 (2H, t, J = 8.7 Hz, 3′,5′-H), 7.33–7.35 (3H, m, Ph), 7.52–7.54 (2H, m, Ph), 7.78 (2H, t, J = 8.7 Hz, 2′,6′-H), 7.88 (1H, d, J = 2.5 Hz, 8-H), 8.15 (1H, d, J = 2.5 Hz, 10-H); 13C-NMR δC (125 MHz, CDCl3) 49.3, 53.7, 85.6, 96.3, 115.6 (d, 2JCF = 21.8 Hz), 118.8, 119.5, 123.0, 123.2, 127.8, 128.3, 128.6, 130.5 (d, 3JCF = 8.5 Hz), 130.8 (d, 4JCF = 3.0 Hz), 131.8, 139.2, 146.3, 153.0, 154.5, 164.0 (d, 1JCF = 250 Hz); MS m/z 444 (100, MH+); HRMS (ES): MH+, found 444.0504. C24H16N379BrF+ requires 444.0512.
9-Bromo-5-(4-chlorophenyl)-7-(phenylethynyl)-2,3-dihydroimidazo[1,2-c]quinazoline (4c). Solid (0.35 g, 77%), Rf (ethyl acetate) 0.47, mp. 170–171 °C; νmax (ATR) 692, 753, 1015, 1072, 1377, 1538, 1574, 1642, 2869 cm−1; 1H-NMR δH (500 MHz, CDCl3) 4.14 (4H, s, -CH2CH2-), 7.33–7.35 (3H, m, Ph), 7.46 (2H, d, J = 8.5 Hz, 3′,5′-H), 7.52–7.54 (2H, m, Ph), 7.72 (2H, d, J = 8.5 Hz, 2′,6′-H), 7.88 (1H, d, J = 2.5 Hz, 8-H), 8.15 (1H, d, J = 2.5 Hz, 10-H); 13C-NMR δC (125 MHz, CDCl3) 49.2, 53.8, 85.5, 96.5, 118.9, 119.5, 123.0, 123.3, 127.8, 128.3, 128.7, 128.8, 129.8, 131.8, 133.0, 136.9, 139.2, 146.2, 152.9, 154.4; MS m/z 460 (100, MH+); HRMS (ES): MH+, found 460.0217. C24H16N335Cl79Br+ requires 460.0217.
9-Bromo-5-phenyl-7-(pyridin-2-ylethynyl)-2,3-dihydroimidazo[1,2-c]quinazoline (4d). Solid (0.37 g, 79%), Rf (ethyl acetate) 0.23, mp. 163–164 °C; νmax (ATR) 445, 525, 699, 774, 879, 1258, 1377, 1422, 1494, 1581, 1641, 1709, 2212, 2869 cm−1; 1H-NMR δH (500 MHz, CDCl3) 4.09 (4H, m, -CH2CH2-), 7.20 (1H, ddd, J = 1.0, 5.0 and 8.0 Hz, 4-H), 7.42–7.48 (4H, m, Ph), 7.62 (1H, dt, J = Hz, 5′′-H), 7.77 (2H, m, Ph), 7.96 (1H, d, J = 2.5 Hz, 8-H), 8.10 (1H, d, J = 2.5 Hz, 10-H), 8.61 (1H, d, J = 3.0 Hz, 3-H); 13C-NMR δC (125 MHz, CDCl3) 49.3, 53.3, 85.6, 99.9, 118.7, 119.5, 122.3, 123.0, 127.6, 128.3, 128.5, 128.7, 130.6, 134.5, 136.1, 140.0, 143.2, 146.9, 149.9, 154.4, 154.5; MS m/z 395 (100, MH+); HRMS (ES): MH+, found 394.0551. C20H16N3O79Br+ requires 394.0555.
9-Bromo-5-(4-fluorophenyl)-7-(pyridin-2-ylethynyl)-2,3-dihydroimidazo[1,2-c]quinazoline (4e). Solid (0.39 g, 83%), Rf (ethyl acetate) 0.21, mp. 201–202 °C; νmax (ATR) 509, 549, 694, 777, 835, 1219, 1380, 1426, 1465, 1581, 1640, 1738, 2209, 2852 cm−1; 1H-NMR δH (500 MHz, CDCl3) 4.12 (4H, s, -CH2CH2-), 7.17 (2H, t, J = 8.7 Hz, 3,5-H), 7.22 (1H, ddd, J = 1.0, 5.0 and 8.0 Hz, 4-H), 7.50 (1H, d, J = 8.0 Hz, 6-H), 7.65 (1H, dt, J = 2.0 and 8.0 Hz, 5-H), 7.76 (2H, t, J = 8.7 Hz, 2,6-H), 7.95 (1H, d, J = 2.5 Hz, 8-H), 8.15 (1H, d, J = 2.5 Hz, 10-H), 8.61 (1H, d, J = 3.0 Hz, 3-H); 13C-NMR δC (125 MHz, CDCl3) 49.3, 53.3, 85.3, 95.0, 115.6 (d, 2JCF = 21.9 Hz), 118.6, 119.6, 122.2, 122.9, 127.4, 128.5, 130.5 (d, 2JCF = 8.5 Hz), 130.7 (d, 4JCF = 3.1 Hz), 135.9, 143.2, 146.6, 150.0, 153.2, 154.2, 156.2, 163.9 (d, 1JCF = 251.4 Hz); MS m/z 445 (100, MH+); HRMS (ES): MH+ found 445.0463. C23H15N479BrF+ requires 445.0464.
9-Bromo-5-(4-chlorophenyl)-7-(pyridin-2-ylethynyl)-2,3-dihydroimidazo[1,2-c]quinazoline (4f). Solid (0.41 g, 85%), Rf (ethyl acetate) 0.26, mp. 214–215 °C; νmax (ATR) 526, 560, 780, 1510, 1093, 1286, 1378, 1421, 1493, 1532, 1580, 1642, 2217, 2870 cm−1; 1H-NMR δH (500 MHz, CDCl3) 4.12 (4H, s, -CH2CH2-) 7.24 (1H, ddd, J = 1.0, 5.0 and 8.0 Hz, 4-H), 7.46 (2H, d, J = 7.8 Hz, 3,5-H), 7.52 (1H, d, J = 8.0 Hz, 6H), 7.66 (1H, dt, J = 2.0 and 8.0 Hz, 5H), 7.73 (2H, d, J = 7.8 Hz, 2,6-H), 7.95 (1H, d, J = 2.5, 8-H), 8.16 (1H, d, J = 2.5 Hz, 10-H), 8.63 (1H, d, J = 30 Hz, 3-H); 13C-NMR δC (125 MHz, CDCl3) 49.2, 53.8, 85.2, 95.0, 118.7, 119.6, 122.2, 122.9, 127.4, 128.5, 128.7, 129.7, 132.9, 136.0, 136.8, 139.7, 143.2, 146.6, 150.0, 153.2, 154.1; MS m/z 461 (100, MH+); HRMS (ES): MH+, found 461.0179. C23H15N435Cl79Br+ requires 461.0169.
4-(9-Bromo-5-phenyl-2,3-dihydroimidazo[1,2-c]quinazolin-7-yl)but-3-yn-1-ol (4g). Solid (0.22 g, 55%), Rf (ethyl acetate) 0.21, mp. 155–157 °C; νmax (ATR) 696, 716, 1065, 1282, 1318, 1378, 1552, 1638, 2872, 2920, 3163 cm−1; 1H-NMR δH (500 MHz, CDCl3) 1.70 (1H, br s, OH), 2.66 (2H, t, J = 5.5 Hz, -CH2C≡), 3.70 (2H, t, J = 5.5 Hz, -CH2O-), 4.03–4.09 (4H, m, -CH2CH2-), 7.48–7.53 (3H, m, 3′,4′,5′-H), 7.62–7.65 (2H, m, 2′,6′-H), 7.71 (1H, d, J = 2.5 Hz, 8-H), 8.10 (1H, d, J = 2.5 Hz, 10-H); 13C-NMR δC (125 MHz, CDCl3) 24.4, 49.2, 53.6, 60.6, 79.8, 95.4, 118.7, 119.5, 123.2, 127.5, 127.9, 128.4, 128.6, 130.7, 134.3, 138.0, 146.9, 154.6; MS m/z 395 (100, MH+); HRMS (ES): MH+, found 394.0551. C20H17N3O79Br+ requires 394.0555.
4-(9-Bromo-5-(4-fluorophenyl)-2,3-dihydroimidazo[1,2-c]quinazolin-7-yl)but-3-yn-1-ol (4h). Solid (0.26 g, 59%), Rf (ethyl acetate) 0.22, mp. 165–167 °C; νmax (ATR) 541, 703, 783, 838, 890, 1065, 1162, 1234, 1378, 1423, 1511, 1608, 1639, 2872, 2922, 3166 cm−1; 1H-NMR δH (500 MHz, CDCl3) 1.50 (1H, s, -OH), 2.68 (2H, t, J = 5.5 Hz, -CH2C≡), 3.72 (2H, t, J = 5.5 Hz, -CH2-O), 4.04–4.13 (4H, m, -CH2CH2-), 7.19 (2H, t, J = 8.5 Hz, 3′,5′-H), 7.66 (2H, t, J = 8.5 Hz, 2′,6′-H), 7.70 (1H, d, J = 2.5 Hz, 8-H), 8.14 (1H, d, J = 2.5 Hz, 10-H); 13C-NMR δC (125 MHz, CDCl3) 24.5, 49.2, 53.7, 60.5, 92.2, 115.8 (d, 2JCF = 21.9 Hz), 118.8, 123.1, 127.5, 128.5, 130.2 (d, 3JCF = 8.6 Hz), 131.9, 132.0 (d, 4JCF = 2.5 Hz), 132.1, 138.1, 146.8, 153.8, 162.9 (d, 1JCF = 246.7 Hz); MS m/z 412 (100, MH+); HRMS (ES): MH+, found 412.0451. C20H16N3O79BrF+ requires 412.0461.
4-(9-Bromo-5-(4-chlorophenyl)-2,3-dihydroimidazo[1,2-c]quinazolin-7-yl)but-3-yn-1-ol (4i). Solid (0.30 g, 68%), Rf (ethyl acetate) 0.22, mp. 176–178 °C; νmax (ATR) 690, 783, 824, 1066, 1092, 1283, 1457, 1640, 2872, 2921, 3173 cm−1; 1H-NMR δH (500 MHz, CDCl3) 2.67 (2H, t, J = 5.5 Hz, -CH2C≡), 3.10 (1H, br s, -OH), 3.70 (2H, t, J = 5.5 Hz, -CH2O-), 4.01–4.12 (4H, m, -CH2CH2-), 7.46 (2H, d, J = 8.7 Hz, 3′,4′,5′-H), 7.59 (2H, d, J = 8.7 Hz, 2′,6′-H), 7.71 (1H, d, J = 2.5 Hz, 8-H), 8.09 (1H, d, J = 2.5 Hz, 10-H); 13C-NMR δC (125 MHz, CDCl3) 24.3, 49.2, 53.6, 60.5, 79.7, 95.3, 119.0, 119.4, 123.1, 127.4, 128.9, 129.4, 132.6, 137.0, 138.0, 146.6, 153.6, 154.0; MS m/z 428 (100, MH+); HRMS (ES): MH+, found 428.0157. C20H16N3O35Cl79Br+ requires 428.0166.
3.5. Typical Procedure for the Suzuki-Miyaura Cross-Coupling of 4a–h
9-(4-Fluorophenyl)-5-phenyl-7-(phenylethynyl)-2,3-dihydroimidazo[1,2-c]quinazoline (5a). A stirred mixture of 4a (0.30 g, 0.70 mmol), PdCl2(PPh3)2 (0.025 g, 0.035 mmol), PCy3 (0.02 g, 0.07 mmol) and K2CO3 (0.15 g, 1.06 mmol) in 3:1 DMF–EtOH (v/v, 15 mL) was purged with argon gas for 30 min. 4-Fluorophenylboronic acid (0.12 g, 0.84 mmol) was added to the mixture using a syringe. The reaction mixture was heated at 100 °C for 2 h and then quenched with an ice-cold water. The product was extracted into chloroform and the combined organic layers were washed with water, dried over Na2SO4, filtered and evaporated under reduced pressure. The residue was purified by column chromatography on silica gel to afford 5a as a yellow solid (0.23 g, 74%), Rf (ethyl acetate) 0.16, mp. 306–307 °C; νmax (ATR) 693, 759, 834, 1225, 1353, 1512, 1538, 1637, 2872, 2964 cm−1; 1H-NMR δH (500 MHz, CDCl3) 4.12–4.19 (4H, m, -CH2CH2-), 7.15 (2H, t, J = 8.5 Hz, 3′,5′-H), 7.32–7.34 (3H, m, Ph), 7.50–7.56 (3H, m, Ph), 7.56–7.58 (2H, m, Ph), 7.67 (2H, t, J = 8.5 Hz, 2′,6′-H), 7.79–7.82 (2H, m, Ph), 8.01 (1H, d, J = 2.0 Hz, 8-H), 8.20 (1H, d, J = 2.0 Hz, 10-H); 13C-NMR δC (125 MHz, CDCl3) 49.3, 53.7, 87.0, 95.2, 115.8 (d, 2JCF 21.8 Hz), 118.7, 121.9, 123.1, 123.5, 128.2, 128.3, 128.4 (3xC), 128.6 (d, 3JCF 8.5 Hz), 130.4, 131.8, 134.9 (d, 4JCF 2.8 Hz), 135.3, 137.7, 146.7, 153.7, 155.7, 162.8 (d, 1JCF 245.6 Hz); MS m/z 442; HRMS (ES): MH+, found 442.1711. C30H21N3F+ requires 442.1720.
5,9-Bis(4-fluorophenyl)-7-(phenylethynyl)-2,3-dihydroimidazo[1,2-c]quinazoline (5b). Solid (0.22 g, 71%), Rf (ethyl acetate) 0.17, mp. 298–299 °C; νmax (ATR) 576, 697, 760, 851, 1156, 1237, 1509, 1604, 1636, 2872, 2962 cm−1; 1H-NMR δH (500 MHz, CDCl3) 4.16 (4H, s, -CH2CH2-), 7.14 (2H, t, J 8.7 Hz, 3′,5′-H), 7.19 (2H, t, J 8.7 Hz, 3′′,5′′-H), 7.33–7.35 (3H, m, Ph), 7.55–7.57 (2H, m, Ph), 7.65 (2H, t, J = 8.7 Hz, 2′,6′-H), 8.82 (2H, t, J = 8.7 Hz, 2′′,6′′-H), 8.00 (1H, d, J = 2.0 Hz, 8-H), 8.17 (1H, d, J = 2.0 Hz, 10-H); 13C-NMR δC (125 MHz, CDCl3) 49.3, 53.8, 86.9, 95.3, 115.6 (d, 2JCF = 21.7 Hz), 115.8 (d, 2JCF = 21.0 Hz), 118.6, 121.9, 123.1, 123.4, 128.3, 128.4, 128.6 (d, 3JCF = 8.6 Hz), 130.7 (d, 3JCF = 8.5 Hz), 131.1 (d, 4JCF = 2.8 Hz), 131.8, 135.2 (d, 4JCF = 3.0 Hz), 135.3, 137.8, 146.5, 152.8, 155.6, 162.8 (d, 1JCF = 247.6 Hz), 163.8 (d, 1JCF = 247.6 Hz); MS m/z 460; HRMS (ES): MH+, found 460.1622. C30H20N3F2+ requires 460.1625.
5-(4-Chlorophenyl)-9-(4-fluorophenyl)-7-(phenylethynyl)-2,3-dihydroimidazo[1,2-c]quinazoline (5c). Solid (0.25 g, 81%), Rf (ethyl acetate) 0.19, mp. 285–286 °C; νmax (ATR) 520, 543, 691, 780, 970, 1090, 1377, 1492, 1537, 1641, 2869, 2927 cm−1; 1H-NMR δH (500 MHz, CDCl3) 4.15 (4H, s, -CH2CH2), 7.13 (2H, t, J = 8.5 Hz, 3′,5′-H), 7.30–7.40 (3H, m, Ph), 7.47 (2H, d, J = 8.5 Hz, 3′′,5′′-H), 7.55–7.571 (2H, m, Ph), 7.64 (2H, t, J = 8.5 Hz, 2′,6′-H), 7.76 (2H, d, J = 8.5 Hz, 2′′,6′′-H), 8.00 (1H, d, J = 2.0 Hz, 8-H), 8.16 (1H, d, J = 2.0 Hz, 10-H); 13C-NMR δC (125 MHz, CDCl3) 49.3, 53.8, 86.9, 95.3, 115.8 (d, 2JCF = 21.9 Hz), 118.6, 121.9, 123.1, 123.3, 128.3, 128.4, 128.6 (d, 3JCF = 7.6 Hz), 128.7, 131.8, 133.3, 135.1 (d, 4JCF = 3.9 Hz), 135.3, 136.7, 137.9, 152.6, 155.5, 162.8 (d, 1JCF = 246.5 Hz); MS m/z 476; HRMS (ES): MH+, found 476.1332. C30H20N3F35Cl+ requires 476.1330.
2,3-Dihydro-9-(4-methoxyphenyl)-5-phenyl-7-(2-phenylethynyl)imidazo[1,2-c]quinazoline (5d). Solid (0.28 g, 88%), Rf (ethyl acetate) 0.20, mp. 275–276 °C; νmax (ATR) 530, 692, 759, 828, 1012, 1180, 1243, 1355, 1432, 1492, 1515, 1540, 1634; 1H-NMR δH (500 MHz, CDCl3) 3.87 (3H, s, -OCH3), 4.13–4.20 (4H, m, -CH2CH2-), 7.00 (2H, d, J = 8.7 Hz, 3′′,5′′-H), 7.30–7.34 (3H, m, ArH), 7.57–7.59 (2H, m, ArH), 7.50–7.52 (3H, m, ArH), 7.65 (2H, d, J = 8.7 Hz, 2′′,6′′-H), 7.80–7.82 (2H, m, ArH), 8.03 (1H, d, J = 1.0 Hz, 8-H), 8.22 (1H, d, J = 1.0 Hz, 10-H); 13C-NMR δC (125 MHz, CDCl3) 49.3, 53.7, 55.4, 87.3, 95.0, 114.3, 118.6, 121.7, 122.6, 123.6, 128.0, 128.2, 128.3, 128.4, 128.5, 130.4, 131.5, 131.8, 135.1, 138.4, 146.2, 153.4, 159.6; MS m/z 454 (100, MH+); HRMS (ES): MH+, found 454.1918. C31H24N3O+ requires 454.1919.
4-(5-Fluorophenyl)-2,3-dihydro-9-(4-methoxyphenyl)-7-(2-phenylethynyl)imidazo[1,2-c]quinazoline (5e). Solid (0.26 g, 81%), Rf (ethyl acetate) 0.18, mp. 292–293 °C; νmax (ATR) 524, 697, 758, 831, 1014, 1113, 1178, 1242, 1285, 1355, 1461, 1513, 1538, 1604, 1635; 1H-NMR δH (500 MHz, CDCl3) 3.86 (3H, s, -OCH3), 4.16 (4H, s, -CH2CH2-), 6.59 (2H, d, J = 8.7 Hz, 3′′,5′′-H), 7.18 (2H, t, J = 8.7 Hz, 3′,5′-H), 7.32–7.34 (3H, m, Ph), 7.54–7.56 (2H, m, Ph), 7.63 (2H, d, J = 8.7 Hz, 2′′,6′′-H), 7.81 (2H, t, J = 8.7 Hz, 2′,6′-H), 8.02 (1H, d, J = 1.0 Hz, 8-H), 8.21 (1H, d, J = 1.0 Hz, 10-H); 13C-NMR δC (125 MHz, CDCl3) 49.4, 53.7, 55.4, 87.1, 95.0, 114.3, 115.6 (d, 2JCF = 22.8 Hz), 121.7, 122.7, 123.5, 128.0, 128.2, 128.3, 129.7, 130.7 (d, 3JCF = 8.6 Hz), 131.5 (d, 4JCF = 3.0 Hz), 131.8, 135.1, 138.5, 146.1, 152.4, 159.6, 164.9 (d, 1JCF = 250.2 Hz); MS m/z 472 (100, MH+); HRMS (ES): MH+, found 472.1819. C31H23N3OF+ requires 472.1825.
9-(4-Fluorophenyl)-2,3-dihydro-5-phenyl-7-(2-pyridin-2-yl)ethynylimidazo[1,2-c]quinazoline (5f). Solid (0.19 g, 72%), Rf (ethyl acetate) 0.25, mp. 254–255 °C; νmax (ATR) 550, 695, 778, 837, 1161, 1234, 1353, 1426, 1466, 1533, 1581, 1640, 1739, 2208, 2869 cm−1; 1H-NMR δH (500 MHz, CDCl3) 4.12–4.16 (4H, m, -CH2CH2-), 7.14 (2H, t, J = 8.7 Hz, 3′,5′-H), 7.22 (1H, ddd, J = 1.0, 5.0 and 8.0 Hz, 4-H), 7.49–7.52 (3H, m, ArH), 7.55 (1H, d, J = 8.0 Hz, 6-H), 7.63–7.65 (2H, m, ArH), 7.66 (1H, dt, J = 2.0 and 8.0 Hz, 5-H), 8.87 (2H, t, J = 8.5 Hz, 2′,6′-H), 8.09 (1H, d, J = 2.0 Hz, 8-H), 8.21 (1H, d, J = 2.0 Hz, 10-H), 8.63 (1H, d, J = 5.0, 3-H); 13C-NMR δC (125 MHz, CDCl3) 49.3, 53.7, 86.9, 94.1, 115.8 (d, 2JCF = 21.9 Hz), 118.7, 120.9, 122.7, 123.8, 127.5, 128.4, 128.5, 128.6 (d, 3JCF = 8.5 Hz), 130.5, 134.8, 135.0 (d, 4JCF = 3.9 Hz), 135.9, 136.0, 137.7, 143.6, 147.0, 150.0, 154.0, 155.5, 162.8 (d, 1JCF = 245.6 Hz); MS m/z 443 (100, MH+); HRMS (ES): MH+, found 443,1670. C29H20N4F+ requires 443.1672.
5,9-Bis(4-fluorophenyl)-2,3-dihydro-7-(2-pyridin-2-yl)ethynylimidazo[1,2-c]quinazoline (5g). Solid (0.19 g, 72%), Rf (ethyl acetate) 0.20, mp. 263–264 °C; νmax (ATR) 777, 836, 1178, 1195, 1384, 1467, 1510, 1606, 1639, 1739, 2209, 2869 cm−1; 1H-NMR δH (500 MHz, CDCl3) 4.15 (4H, s, -CH2CH2-), 7.13 (2H, t, J = 8.5 Hz, 3,5-H), 7.17 (2H, t, J = 8.5 Hz, 3′,5′-H), 7.24 (1H, ddd, J = 1.0, 5.0 and 8.0 Hz, 4-H), 7.54 (1H, d, J = 8.0 Hz, 6-H), 7.63 (2H, t, J = 8.5 Hz, 2,6-H), 7.66 (1H, dt, J = 2.0 and 8.0 Hz, 5-H), 7.81 (2H, t, J = 8.5 Hz, 2′,6′-H), 8.09 (1H, d, J = 2.5 Hz, 8-H), 8.21 (1H, d, J = 2.5 Hz, 10-H), 8.63 (1H, d, J = 5.0 Hz, 3-H); 13C-NMR δC (125 MHz, CDCl3) 49.3, 53.8, 86.8, 94.1, 115.5 (d, 2JCF = 21.9 Hz), 115.8 (d, 2JCF = 21.9 Hz), 118.6, 120.9, 122.8, 123.8, 127.5, 128.5, 128.6 (d, 3JCF = 8.5 Hz), 130.7 (d, 3JCF = 8.5 Hz), 131.0 (d, 4JCF = 3.9 Hz), 135.7 (d, 4JCF = 3.9 Hz), 135.9, 136.0, 137.7, 143.6, 146.9, 150.1, 153.0, 162.8 (d, 1JCF = 245.6 Hz), 163.9 (d, 1JCF = 250.2 Hz); MS m/z 461(100, MH+); HRMS (ES): MH+, found 461.1572. C29H19N4F2+ requires 461.1578.
5-(4-Chlorophenyl)-9-(4-fluorophenyl)-2,3-dihydro-7-(2-pyridin-2-yl)ethynylimidazo[1,2-c]quinazoline (5h). Solid (0.22 g, 85%), Rf (ethyl acetate) 0.30, mp. 251–252 °C; νmax (ATR) 510, 777, 835, 1015, 1091, 1156, 1221, 1514, 1640, 1738, 2213, 2876 cm−1; 1H-NMR δH (500 MHz, CDCl3) 4.14 (4H, s, -CH2CH2-), 7.13 (2H, t, J = 8.5 Hz, 3,5-H), 7.23 (1H, dt, J = 1.0 and 5.0 Hz, 4-H), 7.46 (2H, d, J = 8.5 Hz, 3',5'-H), 7.54 (1H, d, J = 8.0 Hz, 6-H), 7.60 (2H, t, J = 8.5 Hz, 2,6-H), 7.67 (1H, dt, J = 2.0 and 7.5 Hz, 5-H), 7.75 (2H, d, J = 8.5 Hz, 2,6-H), 8.08 (1H, d, J = 2.0 Hz, 8-H), 8.19 (1H, d, J = 2.0 Hz, 10-H), 8.63 (1H, d, J = 4.5 Hz, 3-H); 13C-NMR δC (125 MHz, CDCl3) 49.2, 53.7, 86.8, 94.1, 115.8 (d, 2JCF = 21.8 Hz), 120.9, 122.8, 123.9, 127.4, 128.4, 128.5 (d, 3JCF = 8.6 Hz), 128.6, 128.7, 128.8, 129.7, 129.8, 129.9, 134.9 (d, 4JCF = 3.7 Hz), 136.0, 136.1, 137.8, 143.5, 146.8, 150.1, 162. (d, 1JCF = 246.6 Hz); MS m/z 488 (100, MH+); HRMS (ES): MH+, found 488.1535. C29H19N4F35Cl+ requires 488.1530.
4-(5-(4-Chlorophenyl)-9-(4-fluorophenyl)-2,3-dihydroimidazo[1,2-c]quinazolin-7-yl)but-3-yn-1-ol (5i). Solid (0.22 g, 85%), Rf (ethyl acetate) 0.30, mp. 251–252 °C; νmax (ATR) 537, 777, 835, 1067, 1233, 1355, 1516, 1639, 1738, 2209, 2875 cm−1; 1H-NMR δH (500 MHz, CDCl3) 2.70 (2H, t, J = 6.0 Hz, ≡CCH2-), 3.18 (1H, br s, OH), 3.34 (2H, t, J = 6.0 Hz, -CH2OH), 4.04–4.06 (2H, m, =NCH2-), 4.11–4.15 (2H, m, -CH2N) 7.13 (2H, t, J = 8.5 Hz, 3,5-H), 7.49 (2H, d, J = 8.7 Hz, 3′,5′-H), 7.61 (2H, d, J = 8.5 Hz, 2,6-H), 7.62 (2H, d, J = 8.7 Hz, 2′,6′-H), 7.84 (1H, d, J = 2.5 Hz, 8-H), 8.16 (1H, d, J = 2.5 Hz, 10-H); 13C-NMR δC (125 MHz, CDCl3) 24.4, 49.2, 53.7, 60.7, 81.1, 94.1, 115.8 (d, 2JCF = 21.9 Hz), 118,5, 121.8, 122.8, 128.6 (d, 3JCF = 8.5 Hz), 128.9, 129.5, 132.8, 133.9, 135.1 (d, 4JCF = 3.9 Hz), 136.8, 138.0, 146.9, 153.3, 162.8 (d, 1JCF = 245.6 Hz); MS m/z 444 (100, MH+); HRMS (ES): MH+, found 444.1272. C26H20N3O35ClF+ requires 444.1279.
3.6. Typical Procedure for the One-Pot Sonogashira and Stille Cross-Coupling of 3a–c
9-(Furan-2-yl)-2,3-dihydro-5-phenyl-7-(2-phenylethynyl)imidazo[1.2-c]quinazoline (6a). A stirred mixture of 3a (0.5 g, 1.11 mmol), PdCl2(PPh3)2 (0.04 g, 0.06 mmol), CuI (0.02 g; 0.11 mmol) and K2CO3 (0.23 g, 1.66 mmol) in 3:1 DMF–EtOH (v/v, 15 mL) was purged with argon gas for 30 min. Phenyl acetylene (0.12 g, 1.22 mmol) was added to the mixture using a syringe. The reaction mixture was stirred at room temperature for 18 h and then a solution of 2-(tributylstannyl)furan (0.59 g, 1.6 mmol) in 3:1 DMF–EtOH (5 mL) was added via a syringe. The mixture was heated at 100 °C for 2 h and then quenched with an ice-cold water. The product was extracted into chloroform and the combined organic layers were washed with water, dried over Na2SO4, filtered and evaporated under reduced pressure. The residue was purified by column chromatography on silica gel to afford 6a as a yellow solid (0.36 g, 78%), Rf (ethyl acetate) 0.30, mp. 206‒207 °C; νmax (ATR) 695, 756, 781, 1013, 1089, 1211, 1250, 1351, 1403, 1491, 1532, 1638, 2869 cm−1; 1H-NMR δH (500 MHz, CDCl3) 4.11–4.30 (4H, m, -CH2CH2-), 6.50 (1H. dd, J = 1.5 and 3.0 Hz, 4-H), 6.78 (1H, d, J = 3.0 Hz, 5-H), 7.32–7.34 (3H, m, ArH), 7.74–7.51 (4H, m, 3-H and ArH), 7.55–7.58 (2H, m, ArH), 7.78–7.80 (2H, m, ArH), 8.13 (1H, d, J = 2.0 Hz, 8-H), 8.28 (1H, d, J = 10-H); 13C-NMR δC (125 MHz, CDCl3) 49.3, 53.6, 86.9, 95.2, 106.2, 112.0, 118.6, 119.9, 121.8, 123.5, 128.2, 128.3, 128.4, 128.6, 130.4, 131.8, 132.1, 134.9, 142.6, 146.5, 152.4, 153.4, 155.6; MS m/z 416 (100, MH+); HRMS (ES): MH+, found 416.1748. C28H20N3O+ requires 416.1763.
5-(4-Fluorophenyl)-9-(furan-2-yl)-2,3-dihydro-7-(2-phenylethynyl)imidazo[1,2-c]quinazoline (6b). Solid (0.35 g, 76%), Rf (ethyl acetate) 0.27, mp. 225‒227 °C; νmax (ATR) 693, 716, 734, 755, 1012, 1252, 1385, 1511, 1604, 1641, 2868 cm−1; 1H-NMR δH (500 MHz, CDCl3) 4.15 (4H, s, -CH2CH2-), 6.50 (1H, dd, J = 2.0 and 3.5 Hz, 4-H), 6.77 (1H, d, J = 3.5 Hz, 5-H), 7.18 (2H, t, J = 8.7 Hz, 3′,5′-H), 7.33–7.36 (3H, m, ArH), 7.49 (1H, d, J =2.0 Hz, 3-H), 7.55–7.57 (2H, m, ArH), 7.81 (2H, t, J = 8.7 Hz, 2′,6′-H), 8.12 (1H, d, J = 2.0 Hz, 8-H), 8.26 (1H, d, J = 2.0 Hz, 10-H); 13C-NMR δC (125 MHz, CDCl3) 49.3, 53.6, 86.8, 95.2, 106.2, 111.9, 115.5 (d, 2JCF = 21.8 Hz), 118.4, 119.9, 121.7, 123.4, 128.2, 128.3, 128.7, 130.5 (d, 3JCF = 8.5 Hz), 130.1 (d, 4JCF = 3.0 Hz), 131.7, 132.0, 142.6, 146.2, 152.2, 152.4, 155.5, 163.9 (d, 1JCF = 250.2 Hz); MS m/z 432 (100, MH+); HRMS (ES): MH+, found 432.1516. C28H19N3OF+ requires 432.1512.
5-(4-Chlorophenyl)-9-(furan-2-yl)-2,3-dihydro-7-(2-phenylethynyl)imidazo[1,2-c]quinazoline (6c). Solid (0.39 g, 85%), Rf (ethyl acetate) 0.28, mp. 222‒223 °C; νmax (ATR) 691, 755, 792, 884, 1011, 1386, 1539, 1639, 2866 cm−1; 1H-NMR δH (500 MHz, CDCl3) 4.15 (4H, s, -CH2CH2-), 6.50 (1H, dd, J = 2.0 and 3.5 Hz, 4-H), 6.78 (1H, d, J = 3.5 Hz, 5-H), 7.33–7.35 (3H, m, ArH), 7.47 (2H, d, J = 8.5 Hz, 3′,5′-H), 7.49 (1H, d, J = 2.0 Hz, 3-H), 7.55–7.57 (2H, m, ArH), 7.74 (2H, t, J = 8.5 Hz, 2′,6′-H), 8.11 (1H, d, J = 2.0 Hz, 8-H), 8.26 (1H, d, J = 2.0 Hz, 10-H); 13C-NMR δC (125 MHz, CDCl3) 49.2, 53.7, 86.8, 95.3, 106.4, 112.0, 118.6, 119.9, 121.8, 123.5, 128.2, 128.3, 128.7, 128.8, 129.8, 131.8, 132.1, 132.3, 136.7, 142.7, 146.2, 152.3, 152.4, 155.5; MS m/z 448 (100, MH+); HRMS (ES): MH+, found 448.1223. C28H19N3O35Cl+ requires 448.1217.
4-(5-(4-Fluorophenyl)-9-furan-2-yl)-2,3-dihydroimidazo[1,2-c]quinazolin-7-yl)but-3-yn-1-ol (6d). Solid (0.27 g, 64%), Rf (ethyl acetate) 0.30, mp. 173–174 °C; νmax (ATR) 592, 727, 839, 855, 886, 1015, 1065, 1227, 1514, 1608, 1640, 2875 cm−1; 1H-NMR δH (500 MHz, CDCl3) 2.69 (2H, t, J = 6.0 Hz, -CH2C≡), 3.18 (1H, br s, OH), 3.73 (2H, t, J = 6.0 Hz, -CH2O), 4.04–4.09 (2H, m, -CH2N), 4.11–4.15 (2H, m, -CH2N=), 7.13 (2H, t, J = 8.7 Hz, 3,5-H), 7.48 (2H, d, J = 8.5 Hz, 3′,5′-H), 7.61 (2H, t, J = 8.5 Hz, 2,6-H), 7.62 (2H, d, J = 8.7 Hz, 2′,6′-H), 7.84 (1H, d, J = 2.5 Hz, 8-H), 8.16 (1H, d, J = 2.5 Hz, 10-H); 13C-NMR δC (125 MHz, CDCl3) 24.4, 49.2, 53.7, 60.7, 81.1, 94.1, 115.8 (d, 2JCF = 21.9 Hz), 118.5, 121.7, 122.7, 128.6 (d, 3JCF 8.5 Hz), 128.9, 132.8, 133.9, 135.9 (d, 4JCF 3.8 Hz), 136.8, 138.0, 153.3, 162.8 (d, 1JCF 246.6 Hz); MS m/z 432 (100, MH+); HRMS (ES): MH+, found 432.1516. C20H19N3OF+ requires 432.1512.
4-(5-(4-Chlorophenyl)-9-furan-2-yl)-2,3-dihydroimidazo[1,2-c]quinazolin-7-yl)but-3-yn-1-ol (6e). Solid (0.28 g, 65%), Rf (ethyl acetate) 0.31, mp. 151–153 °C; νmax (ATR) 729, 784, 885, 1014, 1063, 1091, 1267, 1494, 1545, 1600, 2875 cm−1; 1H-NMR δH (500 MHz, CDCl3) 2.69 (2H, t, J = 6.0 Hz, CH2C≡), 3.18 (1H, br s, OH), 3.73 (2H, t, J = 6.0 Hz, -CH2O), 4.03–4.30 (4H, m, -CH2CH2-), 6.49 (1H, dd, J = 2.0 and 3.0 Hz, 4-H), 6.75 (1H, d, J = 3.5 Hz, 5-H), 7.48 (2H, d, J = 8.5 Hz, 3′,5′-H), 7.47 (1H, d, J = 2.0 Hz, 3-H), 7.62 (2H, d, J = 8.5 Hz, 2′,6′-H), 7.95 (1H, d, J = 2.0 Hz, 8-H), 8.23 (1H, d, J = 2.0 Hz, 10-H); 13C-NMR δC (125 MHz, CDCl3) 24.4, 49.2, 53.6, 60.7, 80.9, 94.0, 106.3, 112.0, 112.1, 113.2, 118.5, 119.5, 128.8, 128.9, 129.5, 129.6, 132.9, 136.7, 142.7, 146.5, 152.2, 155.1; MS m/z 416 (100, MH+); HRMS (ES): MH+, found 416.1157. C24H19N3O235Cl+ requires 416.1166.
3.7. Materials and Methods for in Vitro Cytotoxicity Assays
Human breast adenocarcinoma (MCF-7) cells and human cervical cancer (HeLa) cells used in this experiment were obtained from Cellonex (Johannesburg, South Africa). The cells were maintained in Dulbecco’s Modified Eagle’s (DMEM, HyClone, Thermo Scientific, Aalst, Belgium) supplemented with 0.4 mM
l-glutamine and sodium pyruvate and 10% foetal bovine serum (FBS, HyClone, Thermo Scientific). The cells of a sub-confluent culture were harvested using trypsin-EDTA (HyClone, Thermo Scientific) and centrifuged at 200×
g (where g is the relative centrifugal force) for 5 min. and re-suspended in growth medium to 5 × 10
4 cells/mL. A total of 200 µL of the cell suspension was pipetted into each well of columns 2 to 11 of a 96 well culture plate. The same amount of the growth medium was added to wells of column 1 and 12 to maintain humidity and minimize the edge effect. The plates were incubated at 37 °C in a 5% CO
2 incubator overnight until the cells were in the exponential phase of growth. After incubation, the DMEM was aspirated from the cells and replaced with 200 µL of different concentrations of the test samples (0.1–100 µg/mL). Each dilution of the test sample was tested in quadruplicate in each experiment and the experiments were repeated three times. The plates were again incubated for 2 days at 37 °C in a 5% incubator. A negative control (untreated cells) and positive control (cells treated with different concentrations of doxorubicin hydrochloride, Sigma, GmBH, Germany) were included. After incubation, 30 µL of 5 mg/mL MTT, (Sigma) in phosphate buffered saline PBS was added to each well and the plates were incubated for a further 4 h at 37 °C. The medium in each well was then removed and the formazan crystals formed were dissolved by adding 50 µL of DMSO to each well of the plates. The plates were gently shaken until the crystals were dissolved. The amount of MTT reduction was measured immediately by detecting the absorbance using a microplate reader at a wavelength of 570 nm (VersaMax, Molecular Devices, Sunnyvale, CA, USA). The wells in column 1 and 12, containing medium and MTT but no cells was used to blank the microplate reader. The percentage of cell viability was calculated using the formula below:
The LC
50 values (lethal concentration at which 50% of the cells are killed) were calculated as the concentration of the test sample that resulted in 50% reduction of absorbance compared to untreated cells. The intensity of the MTT formazan produced by living metabolically active cells is directly proportional to the number of live cells present [
13].