Isotope Effects on Chemical Shifts in the Study of Intramolecular Hydrogen Bonds
Abstract
:1. Introduction



2. Results and Discussion
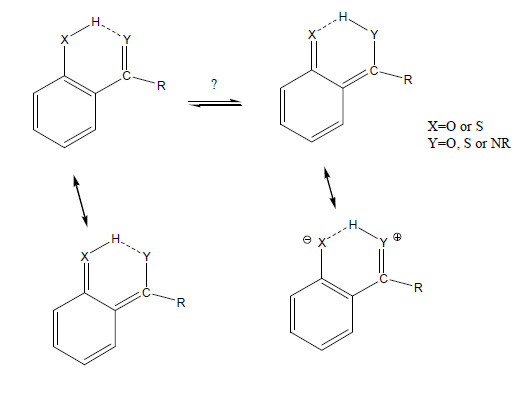
2.1. Isotope Effects of RAHB Cases
2.1.1. Secundary Isotope Effects



| Compound | RN…X in Å a | 2ΔC(ND) in ppm | Refs. |
|---|---|---|---|
| 2,4-dinitro-N,N-(naphthalene-1,8-diyl)bis(2,2,2-trifluoracetamide) See Figure 13 | 2.5968 | 0.32 | [22] |
| 1,4-diaminoanthraquinone | 2.5564 | 0.36 | [23] |
| 1,4-diphenylaminoanthraquinone | 2.5791 | 0.28 | [23] |
| (Z)-N-methyl-3-phenyl-1-amino-3-propa-1-enone | 2.6552 | 0.24 | [24] |
| (Z)-N-phethyl-3-phenyl-1-amino-3-propa-1-enone | 2.6556 | 0.302 | [24] |

| Compound | C=O | C-C | C=C | C-O | O-H | O…O | 2ΔC(OD) a |
|---|---|---|---|---|---|---|---|
| 2,4-di (B) b | 1.2381 | 1.4455 | 1.4205 | 1.3333 | 0.9999 | 2.5980 | 0.302 |
| 2,4-di (B) | 1.2379 | 1.4528 | 1.4255 | 1.3290 | 1.0000 | 2.5878 | 0.332 |
| 2,3-dt (A) | 1.2319 | 1.4593 | 1.4184 | 1.3360 | 0.9909 | 2.6351 | 0.226 |
| 5,6-dimethyl-2,3-dt (C) | 1.2364 | 1.4621 | 1.4066 | 1.3330 | 0.9944 | 2.5727 | 0.303 |


2.1.2. Primary Isotope Effects


2.2. Secondary Isotope Effects of Intramolecular Hydrogen Bonds without RAHB

2.3. Tautomerism
2.3.1. Isotopic Perturbation of Equilibrium



2.3.2. Primary Isotope Effects
Symmetrical Systems

Non-Symmetrical Systems




3. Summary
Conflicts of Interest
References
- Hansen, P.E. NMR studies of Isotope effects of compounds with intramolecular hydrogen bonds. In Isotopes in Chemistry and Biology; Kohen, A., Limbach, H.-H., Eds.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Hansen, P.E. Isotope Effects on Chemical Shifts of Hydrogen Bonded Systems. J. Label. Compd. Radiopharm. 2007, 50, 967–981. [Google Scholar] [CrossRef]
- Hansen, P.E.; Rozwadowski, Z.; Dziembowska, T. Nuclear Magnetic Resonance Spectroscopy of Hydroxy Schiff bases. Curr. Org. Chem. 2009, 13, 194–215. [Google Scholar] [CrossRef]
- Hansen, P.E. Isotope effects on chemical shifts as a tool in the study of tautomeric equilibria. In Tautomerism, Methods and Theories; Antonov, L., Ed.; Wiley: Weinheim, Germany, 2014. [Google Scholar]
- Stare, J.; Jerzierska, A.; Ambrozic, G.; Kosir, I.J.; Kidric, J.; Koll, A.; Mavri, J.; Hadzi, J. Density functional calculation of the 2D potential surface and deuterium isotope effect on C-13 chemical shifts in picolinic acid N-oxide. Comparison with experiment. J. Am. Chem. Soc. 2004, 126, 4437–4443. [Google Scholar] [CrossRef] [PubMed]
- Batiz-Hernandez, H.; Bernheim, R.A. The isotope shift. Prog. NMR Spectrosc. 1967, 3, 63–85. [Google Scholar] [CrossRef]
- Gombler, W. MMR spectroscopic studies on chalcogen compounds. 4. C-13 isotope effects on Se-77 and Te-125 nucelar shieldings and its correlation with C-Se distances-Te-123 isotope effects on Te-125 nuclear shielding. J. Am. Chem. Soc. 1982, 104, 6616–6620. [Google Scholar] [CrossRef]
- Jameson, C.J. The dynamic and electronic factors in isotope effects on NMR parameters. In Isotopes in the Physical and Biomedical Science; Buncel, E., Jones, J.R., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 2, p. 1. [Google Scholar]
- Limbach, H.-H.; Denisov, G.S.; Golubev, N.S. Hydrogen Bond Isotope Effects Studied by NMR in Isotopes in Chemistry and Biology; Kohen, A., Limbach, H.-H., Eds.; CRC: Boca Raton, FL, USA, 2006. [Google Scholar]
- Ubbelohde, A.R.; Gallagher, K.J. Acid-base effects in hydrogen bonds in Crystals. Acta Crystallogr. 1955, 8, 71–83. [Google Scholar] [CrossRef]
- Sharif, S.; Denisov, G.S.; Toney, M.D.; Limbach, H.-H. NMR studies of Solvent-assisted proton transfer in a biologically relevant Schiff Base: Toward a distinction of geometric and equilibrium H-Bond Isotope Effects. J. Am. Chem. Soc. 2006, 128, 3375–3387. [Google Scholar] [CrossRef] [PubMed]
- Filarowski, A.; Hansen, P.E. Secondary Isotope Effects on 13C and 15N Chemical Shifts of Schiff Bases revisited. Z. Phys. Chem. 2013, 227, 917–927. [Google Scholar] [CrossRef]
- O’Leary, D.J.; Hickstein, D.D.; Hansen, B.K.V.; Hansen, P.E. Theoretical and NMR Studies of Deuterium Isotopic Perturbation of Hydrogen Bonding in Symmetrical Dihydroxy Compounds. J. Org. Chem. 2010, 75, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Gilli, G.; Bertulucci, F.; Ferretti, V.; Bertolasi, V. Evidence for resonance-assisted hydrogen bonding from crystal-structure correlations on the enol form of the β-diketone fragment. J. Am. Chem. Soc. 1989, 111, 1023–1028. [Google Scholar] [CrossRef]
- Bertolasi, V.; Pretto, L.; Gilli, G.; Gilli, P. π-Bond cooperativity and anticooperativity effects in resonance-assisted hydrogen bonds (RAHBs). Acta Crystallogr. Sect. B: Struct. Sci. 2006, 62, 850–863. [Google Scholar] [CrossRef]
- Sanz, P.; Mo, O.; Yanez, M.; Elguero, J. Resonance assisted hydrogen bond: A critical examination. Structure and stability of the enols of β-diketones and β-enaminones. J. Phys. Chem. 2007, 111, 3585–3591. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Mo, O.; Yanez, M.; del Bene, J.E. Do coupling constants and chemical shifts provide evidence for the existence of resonance-assisted hydrogen bonds? Mol. Phys. 2004, 23–24, 2563–2574. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Mo, O.; Yanez, M.; del Bene, J.E. Are resonance-assisted hydrogen bonds “resonance” assisted? A theoretical NMR study. Chem. Phys. Lett. 2005, 411, 411–415. [Google Scholar] [CrossRef]
- West-Nielsen, M.; Dominiak, P.M.; Wozniak, K.; Hansen, P.E. Strong intramolecular hydrogen bonding involving nitro- and acetyl groups. Deuterium isotope effects on chemical shifts. J. Mol. Struct. 2006, 789, 81–91. [Google Scholar] [CrossRef]
- Hansen, P.E.; Kamounah, F.S.; Ullah, S. Intramolecular Hydrogen Bonding of o-Hydroxyesters and Related Compounds evaluated by Deuterium Isotope Effects on 13C Chemical Shifts and Principal Component Analysis. J. Mol. Struct. 2007, 844–845, 300–307. [Google Scholar] [CrossRef]
- Hansen, P.E.; Christoffersen, M.; Bolvig, S. Variable Temperature NMR Studies of 2,6-dihydroxy Acylaromatic Compounds. Deuterium Isotope Effects on Chemical Shifts, Isotopic Perturbation of Equilibrium and Barriers to Rotation. Magn. Reson. Chem. 1993, 31, 893–902. [Google Scholar] [CrossRef]
- Piertzak, M.; Grech, E.; Nowicka-Scheibe, J.; Hansen, P.E. Deuterium isotope effects on 13C chemical shifts of negatively charged NH..N systems. Magn. Reson. Chem. 2013, 51, 683–688. [Google Scholar]
- Hansen, P.E.; Kolonicny, A.; Lycka, A. Deuterium isotope effects on 13C nuclear shielding of amino and acetamido compounds. Tautomerism and intramolecular hydrogen bonding. Magn. Reson. Chem. 1992, 30, 786–796. [Google Scholar] [CrossRef]
- Zheglova, D.K.; Genov, D.G.; Bolvig, S.; Hansen, P.E. Deuterium Isotope Effects on 13C Chemical Shifts of Enaminones. Acta Chem. Scand. 1997, 51, 1016–1023. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Hansen, P.E.; Ibsen, N.; Kristensen, T.; Bolvig, S. Deuterium and 18O Isotope Effects on 13C Chemical Shifts of Sterically hindered and/or Intra-molecularly Hydrogen-bonded o-Hydroxy Acyl Aromatics. Magn. Reson. Chem. 1994, 32, 399–408. [Google Scholar] [CrossRef]
- Abildgaard, J.; Bolvig, S.; Hansen, P.E. Unravelling the electronic and vibrational contributions to deuterium isotope effects on 13C chemical shifts using ab initio model calculations. Analysis of the observed isotope effects on sterically perturbed intramolecular hydrogen-bonded o-hydroxy acyl aromatics. J. Am. Chem. Soc. 1998, 120, 9063–9069. [Google Scholar] [CrossRef]
- Hansen, P.E.; Bolvig, S.; Wozniak, K. Steric compression and twist in o-hydroxy acyl aromatics with intramolcular hydrogen bonding. J. Mol. Struct. 2005, 749, 155–168. [Google Scholar] [CrossRef]
- Hansen, P.E.; Kamounah, F.S.; Gryko, D.T. Deuterium isotope effects on 13C chemical shifts of 10-Hydroxybenzo[h]quinolones. Molecules 2013, 18, 4544–4560. [Google Scholar] [CrossRef] [PubMed]
- Ullah, S.; Zhang, W.; Hansen, P.E. Deuterium Isotope Effects on 13C and 15N Chemical Shifts of Intramolecularly Hydrogen-Bonded β-Enamine derivatives of Meldrum’s and Tetronic acid. J. Mol. Struct. 2010, 976, 377–391. [Google Scholar] [CrossRef]
- Jednacak, T.L.; Novak, P.; Uzarevic, I.K.; Bratos, I.; Markovic, J.; Cindric, M. Bioactive phenylenediamine derivatives of dehydroacetic acid: Synthesis, structural characterization and deuterium Isotope effects. Croat. Chem. Acta 2011, 84, 203–209. [Google Scholar] [CrossRef]
- Novak, P.; Piculjan, K.; Bilkjan, T.; Hreanar, T.; Cindric, M.; Rubcik, M.; Meic, Z. Deuterium isotope effects in 13C NMR spectra of intramolecularly hydrogen-bonded salicylaldehyde-4-phenylthiosemicarbazone. Croat. Chem. Acta 2007, 80, 575–581. [Google Scholar]
- Smith, L.B.; Hansen, P.E. Intramolecular Hydrogen Bonding of 5-Acyl-3-methylrhodanines. Z. Phys. Chem. 2008, 222, 1213–1223. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Le, T.N.; Hansen, B.V.K.; Duus, F.; Hansen, P.E. Hydrogen Bonding of Novel o-Hydroxythioacetophenones and related compounds studies by Deuterium Isotope Effects on 13C Chemical Shifts. Magn. Reson. Chem. 2007, 45, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.P.; Nguyen, K.P.P.; Zhang, W.; Kamounah, F.S.; Hansen, P.E. Synthesis and NMR studies of novel hydroxyflavones, hydroxyflavothiones, hydroxyflavanones and hydroxyflavanonethiones. Magn. Reson. Chem. 2009, 47, 1043–1054. [Google Scholar] [CrossRef] [PubMed]
- Dziembowska, T.; Rozwadowski, Z.; Hansen, P.E. Intramolecular Hydrogenbonding of 8-Hydroxyquinoline N-oxides, Quinaldinic Acid N-oxides and Quinaldinamide N-oxide. Deuterium Isotope Effects on 13C Chemical Shifts. J. Mol. Struct. 1997, 436–437, 189–199. [Google Scholar] [CrossRef]
- Guo, J.; Tolstoy, P.M.; Koeppe, B.; Denisov, G.S.; Limbach, H.-H. NMR study of conformational exchange and double-well potential in intramolecular hydrogen bonds in monoanions of succinic acid and derivatives. J. Phys. Chem. 2011, 115, 9828–9836. [Google Scholar] [CrossRef]
- Kato, Y.; Toledo, L.M.; Rebek, J., Jr. Energetics of a low barrier hydrogen bond in nonpolar solvents. J. Am. Chem. Soc. 1996, 118, 8575–8579. [Google Scholar] [CrossRef]
- Tolstoy, P.M.; Schah-Mohammedi, P.; Smirnov, S.N.; Golubev, N.S.; Denisov, G.S.; Limbach, H.-H. Characterization of Fluxional Hydrogen-Bonded Complexes of Acetic Acid and Acetate by NMR: Geometries and Isotope and Solvent Effects. J. Am. Chem. Soc. 2004, 126, 5621–5634. [Google Scholar] [CrossRef] [PubMed]
- Bolvig, S.; Hansen, P.E. Isotope effects on chemical shifts as an analytical tool in structural studies of intramolecular hydrogen bonded compounds. Curr. Org. Chem. 2000, 4, 19–54. [Google Scholar] [CrossRef]
- Lau, J.S.; Perrin, C.L. Isotope effects and symmetry of hydrogen bonds in solution: Single and double-well potential. In Isotope Effects in Chemistry and Biology; Kohen, A., Limbach, H.-H., Eds.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Perrin, C.L.; Burke, K.D. Variable-temperature study of hydrogen-bond symmetry in cyclohexene-1,2-dicarboxylate monoanion in chloroform-d. J. Am. Chem. Soc. 2014, 136, 4355–4362. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.E. Deuterium Isotope Effects on 13C Chemical Shifts of Nitromalonamide. Magn. Reson. Chem. 2008, 46, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.E.; Langgård, M.; Bolvig, S. Isotope Effects on Chemical Shift in Tautomeric Systems with Double Proton transfer. Citrinin. Pol. J. Chem. 1998, 72, 269–276. [Google Scholar]
- Grech, E.; Klimkiewich, J.; Nowicka-Scheibe, J.; Piertzak, M.; Schilff, W.; Pozharski, A.F.; Bolvig, S.; Abildgaard, J.; Hansen, P.E. Deuterium Isotope Effects on 15N, 13C and 1H Chemical Shifts of Proton Sponges. J. Mol. Struct. 2002, 615, 121–140. [Google Scholar] [CrossRef]
- Ajami, D.; Rebek, J., Jr. Coiled Molecules in Spring Loaded Devices. J. Am. Chem. Soc. 2006, 120, 15038–15039. [Google Scholar] [CrossRef]
- Ajami, D.; Tolstoy, P.M.; Dube, H.; Odermatt, S.; Koeppe, B.; Guo, J.; Limbach, H.-H.; Rebek, J., Jr. encapsulated carboxylic acid dimers with compressed hydrogen bonds. Angew. Chem. Int. 2011, 50, 528–531. [Google Scholar] [CrossRef]
- Perrin, C.L.; Kim, Y.-J. Symmetry of the hydrogen bond in malonaldehyde enol in solution. J. Am. Chem. Soc. 1998, 120, 12641–12645. [Google Scholar] [CrossRef]
- Perrin, C.L. Are short, low-barrier hydrogen bonds unusually strong? Acc. Chem. Res. 2010, 43, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Perrin, C.L.; Nielsson, J.B.; Kim, Y.-J. Symmetry of hydrogen bonds in solution, an overview. Ber. Bunsen Ges. 1998, 102, 403–409. [Google Scholar] [CrossRef]
- Bolvig, S.; Hansen, P.E. Deuterium Isotope Effects on 13C Chemical Shifts as a Probe for Tautomerism in Enolic β-Diketones. Magn. Reson. Chem. 1996, 34, 467–478. [Google Scholar] [CrossRef]
- Hansen, P.E.; Sitkowski, J.; Stefaniak, L.; Rozwadowski, Z.; Dziembowska, T. One Bond Deuterium Isotope Effects on 15N Chemical Shifts, 1ΔN(D), in Schiff Bases. Ber. Bunsengesel. Chem. Phys. 1998, 102, 410–413. [Google Scholar] [CrossRef]
- Perrin, C.L. Symmetry of hydrogen bonds in solution. Pure Appl. Chem. 2009, 81, 571–583. [Google Scholar] [CrossRef]
- Bogle, X.S.; Singleton, D.A. Isotope-induced desymmetrization can mimic isotopic perturbation of equilibria. On the symmetry of bromonium ions and hydrogen bonds. J. Am. Chem. Soc. 2011, 133, 17172–17175. [Google Scholar] [CrossRef] [PubMed]
- Perrin, C.L.; Lau, J.S.; Kim, Y.-S.; Kari, P.; Moor, C.; Rheingold, A.L. Asymmetry of the “strongest” OHO hydrogen bond, in the monoanion of (+−)-α,α'-ditert-butylsuccinate. J. Am. Chem. Soc. 2009, 131, 13548–13554. [Google Scholar] [CrossRef] [PubMed]
- Perrin, C.L.; Lau, J.S.; Kim, Y.-S.; Kari, P.; Moor, C.; Rheingold, A.L. Assymmetry of the “strongest” OHO hydrogen bond, in the monoanion of (+−)-α,α'-ditert-butylsuccinate. J. Am. Chem. Soc. 2010, 132, 2099–2100. [Google Scholar] [CrossRef]
- Perrin, C.L.; Kari, P.; Moore, C.; Rheingold, A.L. Hydrogen-bond symmetry in difluoromaleate monanion. J. Am. Chem. Soc. 2012, 134, 7766–7772. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, C.; Lin, J.; Frey, P.A. The deuterium isotope effect on the NMR signal of the low-barrier hydrogen bond in a transition-state analog complex of chymotrypsin. Biochem. Biophys. Res. Commun. 2000, 273, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, P.; Ozeryanskii, V.S.; Sobczyk, L.; Pozharskii, F. Primary H-1/H-2 isotope effect in the NMR chemical shift of HClO4 salts of 1,8-bis(dimethylamino)naphthalene derivatives. J. Phys. Org. Chem. 2007, 20, 643–648. [Google Scholar] [CrossRef]
- Perrin, C.L.; Nielson, J.B. Asymmetry of hydrogen bonds in solutions of monoanions of dicarboxylic acids. J. Am. Chem. Soc. 1997, 119, 12734–12741. [Google Scholar] [CrossRef]
- Hansen, P.E.; Kamounah, F.S.; Zhiryakova, D.; Manolova, Y. Liudmil Antonov, 1,1',1''-(2,4,6-Trihydroxybenzene-1,3,5-triyl)triethanone non-tautomerism. Tetrahdron Lett. 2014, 55, 354–357. [Google Scholar] [CrossRef]
- Serdiuk, I.E.; Wera, M.; Roshal, A.; Sowisnki, P.; Zadykowicz, B.; Blazejowski, I.J. Tautomerism, structure and properties of 1,1'1''-(2,4,6-trihydroxybenzene-1,3,5,tiy)triethanone. Tetrahedron Lett. 2011, 52, 2737–2740. [Google Scholar] [CrossRef]
- Rozwadowski, Z.; Nowak-Wydra, B. Chiral recognition of Schiff bases buy 15N NMR spectroscopy in the presence of a dirhodium complex. Deuterium isotope effect on 15N chemical shift of the optically active Schiff bases and their dirhodium tetracarboxylate adducts. Magn. Reson. Chem. 2008, 45, 974–978. [Google Scholar] [CrossRef]
- Hansen, P.E.; Filarowski, A. Characterisation of the PT-form of o-Hydroxy Acylaromatic Schiff bases by NMR Spectroscopy and DFT Calculations. J. Mol. Struct. 2004, 707, 69–75. [Google Scholar] [CrossRef]
- Filarowski, A.; Koll, A.; Rospenk, M.; Krol-Starzomska, I.; Hansen, P.E. Tautomerism of sterically hindered Schiff bases. Deuterium Isotope Effects on 13C Chemical Shifts. J. Phys. Chem. A 2005, 109, 4464–4473. [Google Scholar] [CrossRef] [PubMed]
- Limbach, H.-H.; Pietzak, M.; Benedict, H.; Tolstoy, P.M.; Golubev, N.S.; Denisov, G.S. Empirical corrections for anharmonic zero-point vibrations of hydrogen and deuterium in geometric hydrogen bond correlations. J. Mol. Struct. 2004, 706, 115–119. [Google Scholar] [CrossRef]
- Dobosz, R.; Skotnicka, A.; Rozwadowski, Z.; Dziembowska, T. Stability of N-(ortho-hydroxynaphtylmethylene)methylamines and their tautomers. J. Mol. Struct. 2010, 979, 194–199. [Google Scholar] [CrossRef]
- Sharif, S.; Denisov, G.S.; Toney, M.D.; Limbach, H.-H. NMR studies of coupled low- and high-barrier hydrogen bonds in pyridoxal-5'-phosphate model systems in polar solution. J. Am. Chem. Soc. 2007, 129, 6313–6327. [Google Scholar] [CrossRef] [PubMed]
- Rozwadowski, Z. Deuterium isotope effect on C-13 chemical shifts of tetrabutylammonium salts of Schiff bases amino acids. Magn. Reson. Chem. 2006, 44, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Rozwadowski, Z. Deuterium isotope effects on C-13 chemical shifts of lithium salts of Schiff bases amino acids. J. Mol. Struct. 2005, 753, 127–131. [Google Scholar] [CrossRef]
- Ossowicz, P.; Janus, E.; Schroeder, G.; Rozwadowski, Z. Spectroscopic studies of amino acids ionic liquid-supported Schiff bases. Molecules 2013, 18, 4986–5004. [Google Scholar] [CrossRef] [PubMed]
- Golubev, N.S.; Smirnov, S.N.; Tolstoy, P.M.; Sharif, S.; Toney, M.D.; Denisov, G.S.; Limbach, H.-H. Observation by NMR of the tautomerism of an intramolecular OHOHN-charge relay chain in a model Schiff base. J. Mol. Struct. 2007, 844–845, 319–327. [Google Scholar] [CrossRef]
- Hansen, P.E.; Kamounah, F.S.; Hansen, B.K.V.; Spanget-Larsen, J. Conformational and tautomeric eccentricites of 2-acetyl-1,8-dihydroxynaphthalenes. Magn. Reson. Chem. 2007, 45, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Hansen, P.E.; Borisov, E.V.; Lindon, J.C. Determination of the Tautomeric Equilibria of Pyridoyl Benzoyl β-Diketones in the Liquid and Solid State through the use of Deuterium Isotope Effects on 1H and 13C NMR Chemical Shifts and Spin Coupling Constants. Spectrochim. Acta 2015, 136, 107–112. [Google Scholar] [CrossRef]
- Mazzini, S.; Merlini, L.; Mondelli, R.; Nasini, G.; Ragg, E.; Scaglioni, L. Deuterium isotope effect on H-1 and C-13 chemical shifts of intramolecularly hydrogen bonded perylenequinones. J. Chem. Soc. Perkin Trans. 2 1997, 2013–2021. [Google Scholar] [CrossRef]
- Bolvig, S.; Hansen, P.E.; Morimoto, H.; Wemmer, D.; Williams, P. Primary tritium and deuterium isotope effects on chemical shifts of compounds having an intramolecular hydrogen bond. Magn. Reson. Chem. 2000, 38, 525–535. [Google Scholar] [CrossRef]
- Khatipov, S.A.; Shapet’ko, N.N.; Bogachev, Y.S.; Andreichov, Y.S. Temperature and the enol proton isotopic-substitution effect on chemical shifts of NMR H-1,C-13 of beta-dicarbonyl compounds with the high intramolecular hydrogen bond. Zhur. Fiz. Khim. 1985, 59, 2097–2099. [Google Scholar]
- Andresen, B.; Duus, F.; Bolvig, S.; Hansen, P.E. Variable temperature 1H and 13C spectroscopic investigation of the enol-enethiol tautomerism of β-thioxoketones. Isotope effects due to deuteron chelation. J. Mol. Spectrosc. 2000, 552, 45–62. [Google Scholar]
- Vasquez, T.E., Jr.; Bergset, J.M.; Fierman, M.B.; Nelson, A.; Roth, J.; Khan, S.I.; O’Leary, D.J. Using Equilibrium Isotope Effects to Detect Intramolecular. OH/OH Hydrogen Bonds: Structural and Solvent Effects. J. Am. Chem. Soc. 2002, 124, 2931–2938. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hansen, P.E. Isotope Effects on Chemical Shifts in the Study of Intramolecular Hydrogen Bonds. Molecules 2015, 20, 2405-2424. https://doi.org/10.3390/molecules20022405
Hansen PE. Isotope Effects on Chemical Shifts in the Study of Intramolecular Hydrogen Bonds. Molecules. 2015; 20(2):2405-2424. https://doi.org/10.3390/molecules20022405
Chicago/Turabian StyleHansen, Poul Erik. 2015. "Isotope Effects on Chemical Shifts in the Study of Intramolecular Hydrogen Bonds" Molecules 20, no. 2: 2405-2424. https://doi.org/10.3390/molecules20022405
APA StyleHansen, P. E. (2015). Isotope Effects on Chemical Shifts in the Study of Intramolecular Hydrogen Bonds. Molecules, 20(2), 2405-2424. https://doi.org/10.3390/molecules20022405