Two New Phenolic Glucosides from Lagerstroemia speciosa
Abstract
:1. Introduction
2. Results and Discussion
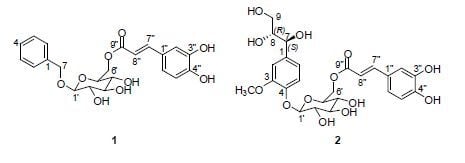
2.1. Structural Elucidation of Isolated Compounds


| 1 | 2 | ||||
|---|---|---|---|---|---|
| Position | δH (J in Hz) | δC | Position | δH (J in Hz) | δC |
| 1 | 137.8 | 1 | 138.7 | ||
| 2,6 | 7.38, d (7.3) | 127.8 | 2 | 7.08, d, (1.8) | 112.5 |
| 3,5 | 7.31, t (7.3) | 128.1 | 3 | 150.8 | |
| 4 | 7.27, t (7.3) | 127.4 | 4 | 147.0 | |
| 7a | 4.76, d (12.1) | 69.6 | 5 | 7.09, d (8.3) | 117.8 |
| 7b | 4.58, d (12.1) | 6 | 6.83, dd (8.3, 1.8) | 120.7 | |
| 1' | 4.28, d (7.9) | 102.0 | 7 | 4.52, d (4.2) | 75.2 |
| 2' | 3.07, m | 73.4 | 8 | 3.62, m | 77.5 |
| 3' | 3.17, m | 76.5 | 9a | 3.48, m | 64.4 |
| 4' | 3.16, m | 70.1 | 9b | 3.35, m | |
| 5' | 3.40, m | 73.8 | 1' | 4.87, d (7.6) | 102.9 |
| 6'a | 4.41, brd (11.7) | 63.5 | 2' | 3.52, m | 75.0 |
| 6'b | 4.19, dd (11.7, 6.6) | 3' | 3.49, m | 77.9 | |
| 1'' | 125.1 | 4' | 3.41, m | 72.0 | |
| 2'' | 7.04, brs | 114.5 | 5' | 3.68, m | 75.8 |
| 3'' | 145.5 | 6'a | 4.53, dd (11.9, 2.1) | 64.8 | |
| 4'' | 149.2 | 6'b | 4.32, dd (11.9, 6.9) | ||
| 5'' | 6.72, d (7.8) | 115.7 | 1'' | 127.9 | |
| 6'' | 6.99, d (7.2) | 121.5 | 2'' | 7.06, d (2.0) | 115.4 |
| 7'' | 7.51, d (15.9) | 145.4 | 3'' | 147.3 | |
| 8'' | 6.30, d (15.9) | 113.4 | 4'' | 149.9 | |
| 9'' | 166.6 | 5'' | 6.80, d (8.2) | 116.8 | |
| 6'' | 6.96, dd (8.2, 2.0) | 123.2 | |||
| 7'' | 7.57, d (15.9) | 147.3 | |||
| 8'' | 6.28, d (15.9) | 115.1 | |||
| 9'' | 169.1 | ||||
| OCH3 | 3.87, s | 56.8 | |||
2.2. Inhibitory Activity against NO Production
| Compound | Cell Viability IC50 (μM) | NO Inhibition IC50 (μM) |
|---|---|---|
| 1 | >100 | 81.8 ± 1.3 |
| 2 | 87.7 ± 3.5 | 71.1 ± 1.9 |
| 3 | >100 | 81.9 ± 1.9 |
| 4 | >100 | 83.3 ± 0.9 |
| 5 | >100 | 69.5 ± 1.4 |
| 6 | 99.0 ± 0.4 | 73.8 ± 0.5 |
| 7 | >100 | 76.2 ± 0.6 |
| 8 | 84.8 ± 5.0 | 70.7 ± 1.2 |
| 9 | >100 | 79.1 ± 1.3 |
| 10 | >100 | 78.0 ± 2.3 |
| 11 | >100 | 81.2 ± 0.7 |
| 12 | >100 | 81.1 ± 0.5 |
3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Enzymatic Hydrolysis of Compound 2
3.5. Measurement of NO Production
3.6. Cell Viability Test
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Klein, G.; Kim, J.; Himmeldirk, K.; Cao, Y.; Chen, X. Antidiabetes and anti-obesity activity of Lagerstroemia speciosa. Evid. Based Complement. Altern. Med. 2007, 4, 401–407. [Google Scholar] [CrossRef]
- Stohs, S.J.; Miller, H.; Kaats, G.R. A review if the efficacy and safety of banaba (Lagerstroemia speciosa L.) and corosolic acid. Phytother. Res. 2012, 26, 317–324. [Google Scholar] [PubMed]
- Hou, W.; Li, Y.; Zhang, Q.; Wei, X.; Peng, A.; Chen, L.; Wei, Y. Triterpene acids isolated from Lagerstroemia speciosa leaves as α-glucosidase inhibitors. Phytother. Res. 2009, 23, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Ragasa, C.Y.; Ngo, H.T.; Rideout, J.A. Terpenoids and sterols from Lagerstroemia speciosa. J. Asian Nat. Prod. Res. 2005, 7, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Nutan; Modi, M.; Goel, T.; Das, T.; Malik, S.; Suri, S.; Rawat, A.K.S.; Srivastava, S.K.; Tuli, R.; Malhotra, S.; et al. Ellagic acid & gallic acid from Lagerstroemia speciosa L. inhibit HIV-1 infection through inhibition of HIV-1 prorotease & reverstranscriptase activity India. J. Med. Res. 2013, 137, 540–548. [Google Scholar]
- Song, J.H.; Park, K.S.; Kwon, D.H.; Choi, H.J. Anti-human rhinovirus 2 activity and mode of action of quercetin-7-glucoside from Lagerstroemia speciosa. J. Med. Food. 2013, 16, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; He, K.; Roller, M.; Zheng, B.; Chen, X.; Shao, Z.; Peng, T.; Zheng, Q. Active compounds from Lagerstroemia speciosa, insulin-like glucose uptake-stimulatory/inhibitory and adipocyte differentiation-inhibitory activities in 3T3-L1 cells. J. Agric. Food Chem. 2008, 56, 11668–11674. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Kim, J.K.; Li, Y.S.; Liu, X.Q.; Li, J.; Chen, X.Z. An extract of Lagerstroemia speciosa L. has insulin-like glucose uptake-stimulatory and adipocyte differentiation-inhibitory activities in 3T3-L1 cells. J. Nutr. 2001, 131, 2242–2247. [Google Scholar] [PubMed]
- Priya, T.T.; Sabu, M.C.; Jolly, C.I. Free radical scavenging and anti-inflammatory properties of Lagerstroemia speciosa (L.). Inflammopharmacology 2008, 16, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, J.; Wang, J. A novel kaemfperol triglycoside from flower buds of Panax quinquefolium. Chem. Nat. Compd. 2009, 45, 808–810. [Google Scholar] [CrossRef]
- Calzada, F.; Cedillo-Rivera, R.; Mata, R. Antiprotozoal activity of the constituents of Conyza filaginoides. J. Nat. Prod. 2001, 64, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Hamerski, L.; Bomm, M.D.; Silva, D.H.; Young, M.C.; Furlan, M.; Eberlin, M.N.; Castro-Gamboa, I.; Cavalheiro, A.J.; da Silva Bolzani, V. Phenylpropanoid glucosides from leaves of Coussarea hydrabgeufolia (Rubiaceae). Phytochemistry 2005, 66, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Isaza, J.H.; Uto, H.; Yoshida, T.A. Flavonol-lignan ester and accompanying acylated, glucoside from Monochaetum multiflorum. Phytochemistry 2001, 58, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Chen, B.; Fu, L.; Wang, M. Chemical constituents from the stems of Dendrobium denneanum (II). Chin. J. Appl. Environ. Biol. 2013, 19, 952–955. [Google Scholar]
- Galland, S.; Mora, N.; Abert-Vian, M.; Rakotomanomana, N.; Dangles, O. Chemical synthesis of hydroxycinnamic acid glucosides and evaluation of their ability to stabilize natural color via anthocyanin copigmentation. J. Agric. Food. Chem. 2007, 55, 7573–7579. [Google Scholar] [CrossRef] [PubMed]
- Hyun, S.K.; Jung, H.A.; Min, B.S.; Jung, J.H.; Choi, J.S. Isolation of phenolics, nucleosides, saccharides and an alkaloid from the root of Aralia cordata. Nat. Prod. Sci. 2010, 16, 20–25. [Google Scholar]
- Gan, M.; Zhang, Y.; Lin, S.; Liu, M.; Song, W.; Zi, J.; Yang, Y.; Fan, X.; Shi, J.; Hu, J.; et al. Glycosides from the root of Iodes cirrhosa. J. Nat. Prod. 2008, 71, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Fujimatu, E.; Kitajima, J. Water-soluble constituents of anis: New clucosides of Anethol glycol and related compounds. Chem. Pharm. Bull. 2002, 50, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Chung, J.H.; Yoon, J.S.; Ha, Y.M.; Bae, S.; Lee, E.K.; Jung, K.J.; Kim, M.S.; Kim, Y.J.; Kim, M.K.; et al. Ginsenoside Rd inhibits the expressions of iNOS and COX-2 by suppressing NF-kappaB in LPS-stimulated RAW264.7 cells and mouse liver. J. Ginseng Res. 2013, 37, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 6, 8–11 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Cho, J.Y.; Choi, S.J.; Jeon, H.; Kim, Y.D.; Htwe, K.M.; Chin, Y.W.; Lee, W.S.; Kim, J.; Yoon, K.D. Two New Phenolic Glucosides from Lagerstroemia speciosa. Molecules 2015, 20, 4483-4491. https://doi.org/10.3390/molecules20034483
Choi J, Cho JY, Choi SJ, Jeon H, Kim YD, Htwe KM, Chin YW, Lee WS, Kim J, Yoon KD. Two New Phenolic Glucosides from Lagerstroemia speciosa. Molecules. 2015; 20(3):4483-4491. https://doi.org/10.3390/molecules20034483
Chicago/Turabian StyleChoi, Janggyoo, Jae Youl Cho, Soo Jung Choi, Heejin Jeon, Young Dong Kim, Khin Myo Htwe, Young Won Chin, Woo Shin Lee, Jinwoong Kim, and Kee Dong Yoon. 2015. "Two New Phenolic Glucosides from Lagerstroemia speciosa" Molecules 20, no. 3: 4483-4491. https://doi.org/10.3390/molecules20034483
APA StyleChoi, J., Cho, J. Y., Choi, S. J., Jeon, H., Kim, Y. D., Htwe, K. M., Chin, Y. W., Lee, W. S., Kim, J., & Yoon, K. D. (2015). Two New Phenolic Glucosides from Lagerstroemia speciosa. Molecules, 20(3), 4483-4491. https://doi.org/10.3390/molecules20034483