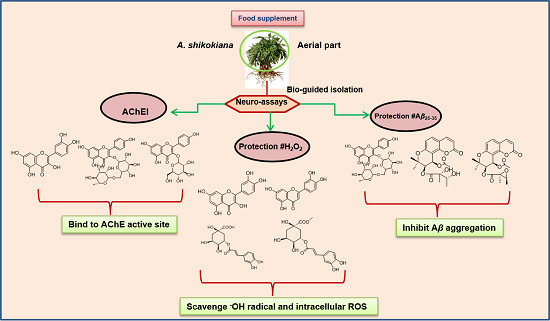
In Vitro Neuroprotective Activities of Compounds from Angelica shikokiana Makino
Abstract
:1. Introduction
2. Results and Discussion

2.1. Acetylcholine Esterase Inhibitory Assay

| Compound | IC50 (µM) | Compound | IC50 (µM) |
|---|---|---|---|
| α-Glutinol (1) | inactive | Luteolin (9) | more than 500 (30% at 250 µM) 3 |
| β-Amyrin (2) | inactive | Methyl chlorogenate (10) | more than 500 (10% at 500 µM) |
| Isoepoxypteryxin (3) | 327.4 ± 3.4 | Chlorogenic acid (11) | inactive |
| Isopteryxin (4) | 475.9 ± 1.5 | Quercetin (12) | 35.5 ± 1.3 |
| β-Sitosterol-3-O-glucoside (5) | inactive | Kaempferol-3-O-glucoside (13) | 80.4 ± 5.1 |
| Hyuganin E (6) | 286.5 ± 2.1 | Kaempferol-3-O-rutinoside (14) | 50.4 ± 0.4 |
| 5-(Hydroxymethyl)-2-furaldehyde (7) | inactive | Adenosine (15) | inactive |
| Kaempferol (8) | more than 500 (30% at 250 µM) 2 |
| Compound | Binding Energy (kcal/mol) | Compound | Binding Energy (kcal/mol) |
|---|---|---|---|
| Quercetin (12) | −129.1 ± 0.7 | Kaempferol-3-O-rutinoside (14) | −195.6 ± 0.3 |
| Kaempferol (8) | −119.3 ± 0.3 | Isoepoxypteryxin (3) | −128.6 ± 0.3 |
| Luteolin (9) | −122.1 ± 0.7 | Isopteryxin (4) | −129.9.2 ± 0.8 |
| Kaempferol-3-O-glucoside (13) | −166.4 ± 0.4 | Hyuganin E (6) | −132.2 ± 0.7 |


2.2. Protection against H2O2-Induced Neurotoxicity and Scavenging of Hydroxyl Radicals and Intracellular ROS

| Sample | IC50 (μM) | |
|---|---|---|
| Non Site-Specific | Site-Specific | |
| Catechin (positive control) | 473 ± 8.6 | 534 ± 19.2 |
| Kaempferol (8) | 720 ± 7.9 | 901 ± 17.9 |
| Luteolin (9) | 753 ± 11.1 | 980 ± 15.8 |
| Methyl chlorogenate (10) | 840 ± 12.4 | 920 ± 10.9 |
| Chlorogenic acid (11) | 602 ± 8.9 | 809 ± 13.7 |
| Quercetin (12) | 672 ± 10.6 | 837 ± 21.6 |
| Kaempferol-3-O-glucoside (13) | 1150 ± 6.7 | 1304 ± 24.3 |
| Kaempferol-3-O-rutinoside (14) | 1030 ± 25.7 | 1540 ± 20.8 |
| Adenosine (15) | - | - |
| Sample | at 300 µM * (% Inhibition) | |
| Non Site-Specific | Site-Specific | |
| α-Glutinol (1) | - | - |
| β-Amyrin (2) | - | - |
| Isoepoxypteryxin (3) | 6 ± 1.4% | - |
| Isopteryxin (4) | 10 ± 2.1% | - |
| β-Sitosterol-3-O-glucoside (5) | - | - |
| Hyuganin E (6) | 37.5 ± 3.5% | - |
| 5-(Hydroxy methyl)-2-furaldehyde (7) | 4 ± 0.3% | - |
2.3. Protection against Aβ25-35 Induced Neurotoxicity and Thioflavin T Assays


3. Experimental Section
3.1. General
3.2. Bio-Guided Isolation
3.3. Acetylcholine Esterase Inhibitory Assay
3.4. Cell Line Assays
3.4.1. Determination of Cell Viability
3.4.2. Protection against H2O2-Induced Neurotoxicity
3.4.3. Protection against Aβ25-35-Induced Neurotoxicity
3.4.4. Scavenging of Intracellular Reactive Oxygen Species (ROS)
3.5. Scavenging of Hydroxyl Radical Using 2-Deoxyribose Degradation Assay
3.6. Thioflavin (ThT) Assay
3.7. Molecular Docking
3.8. Statistical Analysis
| Compound | AchEI IC50(µM) | Protection against H2O2 | Hydroxyl Radical Scavenging/IC50 (µM) | Intracellular ROS Scavenging | Protection against Aβ25-35 | Decrease of ThT Fluorescence |
|---|---|---|---|---|---|---|
| α-Glutinol (1) | - | - | - | - | - | - |
| β-amyrin (2) | - | - | - | - | - | - |
| Isoepoxypteryxin (3) | 327.4 ± 3.4 | - | - | - | ++ | ++ |
| Isopteryxin (4) | 475.9 ± 1.5 | - | - | - | + | + |
| β--Sitosterol-3-O-glucoside (5) | - | - | - | - | - | - |
| Hyuganin E (6) | 286.5 ± 2.1 | + | + | + | ++ | ++ |
| 5-(Hydroxy methyl)-2-furaldehyde (7) | - | - | - | - | - | - |
| Kaempferol (8) | >500 | + | 720 ± 7.9 | + | + | + |
| Luteolin (9) | >500 | ++ | 753 ± 11.1 | ++ | + | - |
| Methyl chlorogenate (10) | >500 | ++ | 840 ± 12.4 | ++ | - | - |
| Chlorogenic acid (11) | - | ++ | 602 ± 8.9 | ++ | - | - |
| Quercetin (12) | 35.5 ± 1.3 | ++ | 672 ± 10.6 | ++ | + | - |
| Kaempferol-3-O-glucoside (13) | 80.4 ± 5.1 | + | 1150 ± 6.7 | + | + | + |
| Kaempferol-3-O-rutinoside (14) | 50.4 ± 0.4 | + | 1030 ± 25.7 | + | ++ | ++ |
| Adenosine (15) | - | - | - | - | - | - |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Talesa, V.N. Acetylcholinesterase in Alzheimer’s disease. Mech. Ageing Dev. 2001, 122, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Racchi, M.; Mazzucchelli, M.; Porrello, E.; Lanni, C.; Govoni, S. Acetylcholinesterase inhibitors: Novel activities of old molecules. Pharm. Res. 2004, 50, 441–451. [Google Scholar] [CrossRef]
- Soto, C.; Braen, M.; Alvarez, J.; Inestrosa, N. Structural determinants of the Alzheimer’s amyloid-β-peptide. J. Neurochem. 1994, 63, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Miranda, S.; Opazo, C.; Larrondo, L.; Munoz, F.; Ruiz, F.; Leighton, F.; Inestrosa, N. The role of oxidative stress in the toxicity induced by amyloid beta-peptide in Alzheimer’s disease. Prog. Neurobiol. 2000, 62, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Selkoe, D. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid β-peptide. Nat. Rev. Mol. Cell Biol. 2007, 2, 101–112. [Google Scholar] [CrossRef]
- Buttereld, D.; Hensley, K.; Cole, P.; Subramaniam, R.; Aksenov, M.; Aksenova, M.; Brummer, P.; Haley, B.; Carney, J. Oxidatively induced structural alteration of glutamine synthetase assessed by analysis of spin label incorporation kinetics: Relevance to Alzheimer’s disease. J. Neurochem. 1997, 68, 2451–2457. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, R. Metal ion-catalyzed oxidation of proteins: Biochemical mechanism and biological consequences. Free Radic. Biol. Med. 1990, 9, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Okuda, H. Effects of active compounds isolated from Angelica shikokiana on lipid metabolism in fat cells. J. Ethnopharm. 1989, 25, 269–280. [Google Scholar] [CrossRef]
- Okuda, H. Pharmaceutical Composition Comprises Extract of Angelica shikokiana Makiao belonging to Umbelliferae. JP 60260520 A. 23 December 1985. [Google Scholar]
- Kurumaya, S.; Kurumaya, S. A Health Tea Prepared from Sweet Potato (Ipomoea batatas, Ipomea batatas), Pueraria lobata Ohwi, and Angelica shikokiana, and Its Preparation Method. JP 2006136229A. 1 June 2006. [Google Scholar]
- Mira, A.; Tanaka, A.; Tateyama, Y.; Kondo, R.; Shimizu, K. Comparative Biological Study of Roots, Stems, Leaves, and Seeds of Angelica shikokiana Makino. J. Ethnopharm. 2013, 148, 980–987. [Google Scholar] [CrossRef]
- Park, S.; Jung, J.; Lee, H.; Lee, Y.; Kim., D.; Kim, J.; Hong, J. The memory ameliorating effects of INM-176, an ethanolic extract of Angelica gigas, against scopolamine- or Aβ1–42-induced cognitive dysfunction in mice. J. Ethnopharm. 2012, 143, 611–620. [Google Scholar] [CrossRef]
- Moon, Y.; Lee, J.; Oh, M.; Pak, Y.; Park, K.; Oh, T.; Yune, T. Inhibition of inflammation and oxidative stress by Angelica dahuricae radix extract decreases apoptotic cell death and improves functional recovery after spinal cord injury. J. Neurosci. Res. 2012, 90, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, M.; Serang, R.; Mohdhashim, N.; Gwendoline, M.; Ali, A.; Ismail, H. Alkaloids and Sulphur-containing Amides from Glycosmis citrifolia and Glycosmis elongate. Sains Malaysiana 2010, 39, 445–451. [Google Scholar]
- Abbas, M.; Disil, M.; Al-Khalil, S. Isolation and Identification of Anti-Ulcer Components from Anchusa Strigosa Root. Jordan J. Pharm. Sci. 2009, 2, 131–139. [Google Scholar]
- Morikawa, T.; Matsuda, H.; Ohgushi, T.; Nishida, N.; Ishiwada, T. Absolute stereostructures of acylated khellactone-type coumarins from Angelica furcijuga. Heterocycles 2004, 63, 2211–2215. [Google Scholar] [CrossRef]
- Liu, H.; Mou, Y.; Zhao, H.; Wang, J.; Zhou, L.; Wang, M. Flavonoids from Halostachys caspica and Their Antimicrobial and Antioxidant Activities. Molecules 2010, 15, 7933–7945. [Google Scholar] [CrossRef] [PubMed]
- Bylka, W.; Matlawska, I. Flavonoids from Aquilegia vulgaris L. flowers. Acta Polonia. Pharm. Drug Res. 1999, 56, 241–244. [Google Scholar]
- Song, N.; Xu, W.; Guan, H.; Liu, X.; Wang, Y.; Nie, X. Several flavonoids from Capsella bursa-pastoris (L.). Medic. Asian. J. Trad. Med. 2007, 2, 218–222. [Google Scholar]
- Zhu, X.; Dong, X.; Wang, Y.; Ju, P.; Ino, S. Phenolic compounds from Vibrnum cylindricum. Helv. Chem. Acta 2005, 88, 339–342. [Google Scholar] [CrossRef]
- Hyun, S.; Jung, H.; Min, B.; Jung, J.; Choi, J. Isolation of Phenolics, Nucleosides, Saccharides and an Alkaloid from the root of Aralia cordata. Nat. Prod. Sci. 2010, 16, 20–25. [Google Scholar]
- Yuan, Z.; Tezuka, Y.; Fan, W.; Kadota, S.; Lia, X. Constituents of the Underground Parts of Glehnia littoralis. Chem. Pharm. Bull. 2002, 50, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Toriizuka, K.; Nishiyama, P.; Adachi, I.; Kawashiri, N.; Ueno, M.; Terasawa, K.; Horikoshi, H. Isolation of a platelet aggregation inhibitor from Angelica radix. Chem. Pharm. Bull. 1986, 34, 5011–5015. [Google Scholar] [PubMed]
- Sussman, J.; Harel, M.; Frolow, F.; Oefner, C.; Goldman, A.; Toker, L.; Silman, I. Atomic structure of acetylcholinesterase from Torpedo Californica: A prototypic acetylcholine-binding protein. Science 1991, 253, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Harel, M.; Schalk, I.; Ehret-Sabatier, L.; Bouet, F.; Goeldner, M.; Hirth, C.; Axelsen, P.; Silman, I.; Sussman, J. Quaternary ligand binding to aromatic residues in the active-site gorge of acetylcholinesterase. Proc. Natl. Acad. Sci. USA 1993, 90, 9031–9035. [Google Scholar] [CrossRef] [PubMed]
- Shafferman, A.; Velan, B.; Ordentlich, A.; Kronman, C.; Grosfeld, H.; Leitner, M.; Flashner, Y.; Cohen, S.; Barak, D.; Ariel, N. Substrate inhibition of acetylcholinesterase: Residues affecting signal transduction from the surface to the catalytic center. EMBO J. 1992, 11, 3561–3568. [Google Scholar] [PubMed]
- Paul, H.; Axelsen, T.; Chal, H.; Israel, S.; Joel, L. Structure and dynamics of the active site gorge of acetylcholinesterase: Synergistic use of molecular dynamics simulation and X-ray crystallography. Prot. Sci. 1994, 3, 188–197. [Google Scholar]
- Zhang, Y.; Kua, J.; McCammon, J. Role of the Catalytic Triad and Oxyanion Hole in Acetylcholinesterase Catalysis: An ab initio QM/MM Study. J. ACS 2002, 124, 10572–10577. [Google Scholar]
- Kontush, A. Amyloid-β: An antioxidant that becomes a pro-oxidant and critically contributes to Alzheimer’s disease. Free Radic. Biol. Med. 2001, 62, 1120–1131. [Google Scholar] [CrossRef]
- Sinha, M.; Bhowmick, P.; Banerjee, A.; Chakrabarti, S. Antioxidant role of amyloid β protein in cell-free and biological systems: Implication for the pathogenesis of Alzheimer’s disease. Free Radic. Biol. Med. 2013, 56, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Smid, S.; Maag, J.; Musgrave, I. Dietary polyphenol-derived protection against neurotoxic β-amyloid protein: From molecular to clinical. Food Funct. 2012, 3, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Sharoar, M.; Thapa, A.; Shahnawaz, M.; Ramasamy, V.; Woo, E.; Shin, S.; Park, S. Kaempferol-3-O-rhamnoside abrogates amyloid beta toxicity by modulating monomers and remodeling oligomers and fibrils to non-toxic aggregates. J. Biomed. Sci. 2012, 19, 104. [Google Scholar] [CrossRef] [PubMed]
- Soreq, H.; Seidman, S. Acetylcholinesterase—new roles for an old actor. Nat. Rev. Neurosci. 2001, 2, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Brunhofer, G.; Fallarero, A.; Karlsson, D.; Batista, A.; Shinde, P.; Mohan, G.; Vuorela, P. Exploration of natural compounds as sources of new bifunctional scaffolds targeting cholinesterases and beta amyloid aggregation: The case of chelerythrine. Bioorg. Med. Chem. 2012, 20, 6669–6679. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.; Lourtney, D.; Andres, V.; Gmelin, G. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharm. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Kumar, V.; Mal, M.; Houghton, P. In vitro acetylcholine esterase inhibitory activity of essential oil and its main constituents of Acorus calamus. Planta Med. 2007, 73, 283–285. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, L.; Yu, H.; Sun, Z.; Lin, X.; Tan, C.; Lu, R. Curcumin protects mouse neuroblastoma Neuro-2A cells against hydrogen-peroxide-induced oxidative stress. Food Chem. 2011, 129, 387–394. [Google Scholar] [CrossRef]
- Tan, H.; Wong, D.; Ling, S.; Chuah, C.; Kadir, H. Neuroprotective activity of galloylated cyanogenic glucosides and hydrolysable tannins isolated from leaves of Phyllagathis rotundifolia. Fitoterapia 2012, 83, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lee, Y.; Kwak, J.; Na, Y.; Yoon, H. Protective effects of a chalcone derivative against Aβ-induced oxidative stress and neuronal damage. BMB Rep. 2011, 44, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Nishimura, T.; Teruya, K. Protective mechanism of reduced water against alloxan-induced pancreatic β-cell damage: Scavenging effect against reactive oxygen species. Cytotechnology 2002, 40, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Raman, C.; Rajesh, A.; Ravinder, K.; Shikha, S.; Satish, C.; Purib, R.; Surender, S.; Ashok, K.; Sharmaa, J.; Haider, A.; et al. 3-O-β-d-Galactopyranoside of Quercetin as an Active Principle from High Altitude Podophyllum hexandrum and Evaluation of its Radioprotective Properties. Z. Naturforsch. 2005, 60c, 728–738. [Google Scholar]
- Rima, J.; Li, Q.; Aouezova, L. Generation of Free Radicals, Analytical Methods, Bacterial Disinfections, and Oxidative Destruction of Organic Chemicals Using Zero Valent Iron and Other Metals. U.S. Patent 8048317 B2, 1 November 2011. [Google Scholar]
- Xican, L. Solvent effects and improvements in the deoxyribose degradation assay for hydroxyl radical-scavenging. Food Chem. 2013, 141, 2083–2088. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.; Burke, R.; Doig, A. Inhibition of toxicity in the β-Amyloid peptide fragment β-(25–35) using N-Methylated derivatives: A general strategy to prevent amyloid formation. J. Biol. Chem. 2000, 275, 5109–25115. [Google Scholar]
- Li, L.; Hyung, H.; Young, T.; Matthew, P. Discovery of amyloid-beta aggregation inhibitors using an engineered assay for intracellular protein folding and solubility. Prot. Sci. 2008, 18, 277–286. [Google Scholar]
- Hai-Bang, T.; Shimizu, K. Potent angiotensin-converting enzyme inhibitory tripeptides identified by a computer-based approach. J. Mol. Graphics Mod. 2014, 3, 206–211. [Google Scholar] [CrossRef]
- Ratnavali, G.; Devi, N.; Sri, K.; Raju, J.; Sirisha, B.; Kavitha, R. An attempt to screen top colorectal cancer drugs by using Molegro Virtual Docker. Ann. Biol. Res. 2011, 2, 114–126. [Google Scholar]
- Sample Availability: Samples of the compounds 1 to 15 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mira, A.; Yamashita, S.; Katakura, Y.; Shimizu, K. In Vitro Neuroprotective Activities of Compounds from Angelica shikokiana Makino. Molecules 2015, 20, 4813-4832. https://doi.org/10.3390/molecules20034813
Mira A, Yamashita S, Katakura Y, Shimizu K. In Vitro Neuroprotective Activities of Compounds from Angelica shikokiana Makino. Molecules. 2015; 20(3):4813-4832. https://doi.org/10.3390/molecules20034813
Chicago/Turabian StyleMira, Amira, Shuntaro Yamashita, Yoshinori Katakura, and Kuniyoshi Shimizu. 2015. "In Vitro Neuroprotective Activities of Compounds from Angelica shikokiana Makino" Molecules 20, no. 3: 4813-4832. https://doi.org/10.3390/molecules20034813
APA StyleMira, A., Yamashita, S., Katakura, Y., & Shimizu, K. (2015). In Vitro Neuroprotective Activities of Compounds from Angelica shikokiana Makino. Molecules, 20(3), 4813-4832. https://doi.org/10.3390/molecules20034813