Zinc Complexes Containing Coumarin-Derived Anilido-Aldimine Ligands as Catalysts for Ring Opening Polymerization of L-Lactide
Abstract
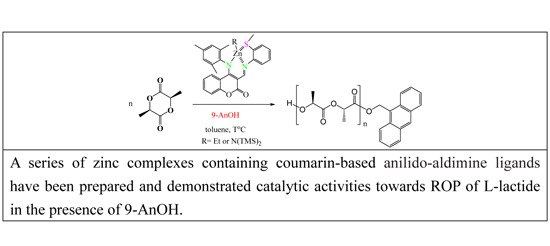
:1. Introduction
2. Results and Discussion
2.1. Syntheses and Characterization of Ligand Precursors and Zinc Complexes



| 1 a | |||
| Zn-N(1) | 2.0650(17) | N(1)-C(6) | 1.310(3) |
| Zn-N(2) | 2.0256(17) | N(2)-C(10) | 1.290(3) |
| Zn-C(29) | 1.987(2) | C(6)-C(7) | 1.444(3) |
| Zn-O(2A) | 2.1062(16) | C(7)-C(10) | 1.437(3) |
| N(2)-Zn-N(1) | 90.95(7) | C(29)-Zn-O(2A) | 106.29(8) |
| C(29)-Zn-N(1) | 132.97(9) | N(1)-Zn-O(2A) | 92.54(6) |
| C(29)-Zn-N(2) | 124.07(8) | N(2)-Zn-O(2A) | 103.41(7) |
| 7 b | |||
| Zn-N(1) | 2.0314(19) | N(1)-C(6) | 1.316(3) |
| Zn-N(2) | 1.9932(19) | N(2)-C(10) | 1.300(3) |
| Zn-N(3) | 1.916(2) | C(6)-C(7) | 1.442(3) |
| Zn-S | 2.6488(6) | C(7)-C(10) | 1.419(3) |
| N(2)-Zn-N(1) | 92.30(8) | N(3)-Zn-S | 106.68(6) |
| N(3)-Zn-N(2) | 128.82(8) | N(2)-Zn-S | 76.57(6) |
| N(3)-Zn-N(1) | 120.25(8) | N(1)-Zn-S | 125.91(5) |

2.2. Polymerization Studies

| Entry | Catalyst | {[L-LA]0:[Zn]0}:[9-AnOH] | Time (min) | Conv. (%) b | Mn(calcd) c | Mn(obsd) d | Mw/Mn d |
|---|---|---|---|---|---|---|---|
| 1 e | 1 | 50:1 | 30 | 65 | 5800 | 3700 | 1.09 |
| 2 e | 2 | 50:1 | 30 | 25 | 1700 | - | - |
| 3 e | 3 | 50:1 | 30 | 90 | 6700 | 4700 | 1.12 |
| 4 e | 4 | 50:1 | 30 | 16 | 1600 | - | - |
| 5 e | 5 | 50:1 | 30 | 5 | 600 | - | - |
| 6 e | 6 | 50:1 | 30 | 43 | 3400 | - | - |
| 7 e,f | 3 | 50:1 | 30 | 10 | 1000 | ||
| 8 e | 3 | 50:1(IPA) | 30 | 90 | 6500 | 5800 | 1.12 |
| 9 e | 3 | 50:1(BnOH) | 30 | 67 | 4900 | 2700 | 1.09 |
| 10 e | 3 | 150:1 | 120 | 93 | 20300 | 23300 | 1.06 |
| 11 e | 3 | 150:1(IPA) | 120 | 86 | 18600 | 22800 | 1.09 |
| 12 | 3 | 50:1 | 15 | 90 | 6700 | 5400 | 1.13 |
| 13 | 3 | 100:1 | 30 | 95 | 13900 | 9600 | 1.06 |
| 14 | 3 | 200:1 | 60 | 97 | 28000 | 19800 | 1.27 |
| 15 | 3 | 300:1 | 90 | 97 | 42200 | 30600 | 1.21 |
| 16 | 3 | 400:1 | 240 | 96 | 55600 | 37300 | 1.23 |
| 17 | 3 | 400:2 | 180 | 94 | 27300 | 20100 | 1.11 |
| 18 | 3 | 400:4 | 90 | 97 | 14200 | 13900 | 1.12 |
| 19 | 3 | 400:8 | 45 | 95 | 7100 | 7700 | 1.10 |
| 20 | 3 | 400:10 | 30 | 95 | 5700 | 6500 | 1.13 |
| 21 | 3 | 400:20 | 15 | 90 | 2800 | 3000 | 1.10 |
| 22 | 3 | 400:40 | 8 | 95 | 1600 | 1600 | 1.09 |
| 23 | 7 | 50:1 | 3 | 91 | 6700 | 5600 | 1.09 |
| 24 | 7 | 100:1 | 6 | 94 | 13700 | 11500 | 1.08 |
| 25 | 7 | 200:1 | 12 | 97 | 28100 | 22500 | 1.08 |
| 26 | 7 | 300:1 | 18 | 97 | 42100 | 32500 | 1.07 |
| 27 | 7 | 400:1 | 24 | 96 | 55500 | 38600 | 1.08 |
| 28 | 7 | 500:1 | 30 | 92 | 66500 | 45400 | 1.06 |
| 29 | 7 | 800:1 | 48 | 94 | 108600 | 57800 | 1.05 |
| 30 | 7 | 50:0 | 15 | 90 | 6600 | 26300 | 1.24 |



3. Experimental Section
3.1. General Information
3.2. Preparations
3.3. Crystal Structure Data
| 1 | 7 | |
|---|---|---|
| Formula | C30H32N2O2Zn | C32H41N3O2SSi2Zn |
| Fw | 517.95 | 653.29 |
| T, K | 150(2) | 150(2) |
| Crystal system | Orthorhomic | Triclinic |
| Space group | P212121 | P-1 |
| a, Å | 9.4413(2) | 10.1352(3) |
| b, Å | 12.7962(2) | 11.1435(5) |
| c, Å | 22.3768(4) | 14.9829(5) |
| α° | 90 | 78.698(3) |
| β° | 90 | 82.913(3) |
| γ° | 90 | 89.969(3) |
| V, Å3 | 2703.40(9) | 1646.21(10) |
| Z | 4 | 2 |
| ρcalc, Mg/m3 | 1.273 | 1.318 |
| μ (Mo Kα), mm−1 | 0.936 | 0.915 |
| Reflections collected | 29922 | 18473 |
| No. of parameters | 316 | 370 |
| R1 a | 0.0328 | 0.0420 |
| w R2 a | 0.0912 | 0.1255 |
| GoF b | 1.004 | 1.000 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Uhrich, K.E.; Cannizzaro, S.M.; Langer, R.S.; Shakesheff, K.M. Polymeric systems for controlled drug release. Chem. Rev. 1999, 99, 3181–3198. [Google Scholar] [CrossRef] [PubMed]
- Dechy-Cabaret, O.; Martin-Vaca, B.; Bourissou, D. Controlled ring-opening polymerization of lactide and glycolide. Chem. Rev. 2004, 104, 6147–6176. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.K. Synthesis of functionalized biodegradable polyesters. Chem. Soc. Rev. 2007, 36, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Tong, R.; Cheng, J. Paclitaxel-initiated, controlled polymerization of lactide for the formulation of polymeric nanoparticulate delivery vehicles. Angew. Chem. Int. Ed. 2008, 47, 4830–4834. [Google Scholar] [CrossRef]
- O’Keefe, B.J.; Hillmyer, M.A.; Tolman, W.B. Polymerization of lactide and related cyclic esters by discrete metal complexes. J. Chem. Soc. Dalton Trans. 2001, 2215–2224. [Google Scholar] [CrossRef]
- Wu, J.; Yu, T.L.; Chen, C.T.; Lin, C.C. Recent developments in main group metal complexes catalyzed/initiated polymerization of lactides and related cyclic esters. Coord. Chem. Rev. 2006, 250, 602–626. [Google Scholar] [CrossRef]
- Platel, R.H.; Hodgson, L.M.; Williams, C.K. Biocompatible Initiators for Lactide Polymerization. Polym. Rev. 2008, 48, 11–63. [Google Scholar] [CrossRef]
- Wheaton, C.A.; Hayes, P.G.; Ireland, B.J. Complexes of Mg, Ca and Zn as homogeneous catalysts for lactide polymerization. Dalton Trans. 2009, 4832–4846. [Google Scholar] [CrossRef]
- Stanford, M.J.; Dove, A.P. Stereocontrolled ring-opening polymerisation of lactide. Chem. Soc. Rev. 2010, 39, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Sutar, A.K.; Maharana, T.; Dutta, S.; Chen, C.T.; Lin, C.C. Ring-opening polymerization by lithium catalysts: an overview. Chem. Soc. Rev. 2010, 39, 1724–1746. [Google Scholar] [CrossRef] [PubMed]
- Ajellal, N.; Carpentier, J.F.; Guillaume, C.; Guillaume, S.M.; Helou, M.; Poirier, V.; Sarazin, Y.; Trifonov, A. Metal-catalyzed immortal ring-opening polymerization of lactones, lactides and cyclic carbonates. Dalton Trans. 2010, 39, 8363–8376. [Google Scholar] [CrossRef] [PubMed]
- Doyle, D.J.; Gibson, V.C.; White, A.J.P. Synthesis and structures of bimetallic and polymeric zinc coordination compounds supported by salicylaldiminato and anilido–aldimine ligands. Dalton Trans. 2007, 358, 358–363. [Google Scholar] [CrossRef]
- Shang, X.; Liu, X.; Cui, D. Yttrium bis(alkyl) and bis(amido) complexes bearing N,O-multidentate ligands. Synthesis and catalytic activity towards ring-opening polymerization of l-lactide. J. Polym. Sci. Part A: Polym. Chem. 2007, 45, 5662–5672. [Google Scholar] [CrossRef]
- Gao, W.; Cui, D.; Liu, X.; Zhang, Y.; Mu, Y. Rare-earth metal bis(alkyl)s supported by a quinolinyl anilido-imine ligand: synthesis and catalysis on living polymerization of ε-caprolactone. Organometallics 2008, 27, 5889–5893. [Google Scholar] [CrossRef]
- Yao, W.; Mu, Y.; Gao, A.; Gao, W.; Ye, L. Bimetallic anilido-aldimine Al or Zn complexes for efficient ring-opening polymerization of ε-caprolactone. Dalton Trans. 2008, 3199–3206. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Lin, C.H.; Lin, C.C.; Ko, B.T. Tridentate anilido-aldimine magnesium and zinc complexes as efficient catalysts for ring-opening polymerization of ε-caprolactone and l-lactide. J. Polym. Sci. Part A: Polym. Chem. 2009, 47, 4927–4936. [Google Scholar] [CrossRef]
- Liu, Y.C.; Lin, C.H; Ko, B.T.; Ho, R.M. Ring-opening polymerization of β-butyrolactone catalyzed by efficient magnesium and zinc complexes derived from tridentate anilido-aldimine ligand. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 5339–5347. [Google Scholar] [CrossRef]
- Allan, L.E.N.; Bélanger, J.A.; Callaghan, L.M.; Cameron, D.J.A.; Decken, A.; Shaver, M.P. Anilido-aldimine aluminum complexes: Synthesis, characterization and lactide polymerization. J. Organomet. Chem. 2012, 706–707, 106–112. [Google Scholar] [CrossRef]
- Wang, C.H.; Li, C.Y.; Huang, B.H.; Lin, C.C.; Ko, B.T. Synthesis and structural determination of zinc complexes based on an anilido-aldimine ligand containing an O-donor pendant arm: Zinc alkoxide derivative as an efficient initiator for ring-opening polymerization of cyclic esters. Dalton Trans. 2013, 42, 10875–10884. [Google Scholar] [CrossRef] [PubMed]
- Lacy, A.; O’Kennedy, R. Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr. Pharm. Des. 2004, 10, 3797–3811. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.V.; Kulkarni, G.M.; Lin, C.H.; Sun, C.M. Recent advances in coumarins and 1-azacoumarins as versatile biodynamic agents. Curr. Med. Chem. 2006, 13, 2795–2818. [Google Scholar] [CrossRef] [PubMed]
- Musa, M.A.; Cooperwood, J.S.; Khan, M.O.F. A review of coumarin derivatives in pharmacotherapy of breast cancer. Curr. Med. Chem. 2008, 15, 2664–2679. [Google Scholar] [CrossRef] [PubMed]
- Grazul, M.; Budzisz, E. Biological activity of metal ions complexes of chromones, coumarins and flavones. Coord. Chem. Rev. 2009, 253, 2588–2598. [Google Scholar] [CrossRef]
- Wagner, B.D. The use of coumarins as environmentally-sensitive fluorescent probes of heterogeneous inclusion systems. Molecules 2009, 14, 210–237. [Google Scholar] [CrossRef] [PubMed]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Kricheldorf, H.R.; Berl, M.; Scharnagl, N. Poly(1actones). 9. Polymerization mechanism of metal alkoxide initiated polymerizations of lactide and various lactones. Macromolecules 1988, 21, 286–293. [Google Scholar] [CrossRef]
- Dubois, P.; Jacobs, C.; Jérôme, R.; Teyssié, P. Macromolecular engineering of polylactones and polylactides. 4. Mechanism and kinetics of lactide homopolymerization by aluminum isopropoxide. Macromolecules 1991, 24, 2266–2270. [Google Scholar] [CrossRef]
- Silvernail, C.M.; Yao, L.J.; Hill, L.M. R.; Hillmyer, M.A.; Tolman, W.B. Structural and mechanistic studies of bis(phenolato)amine zinc(II) catalysts for the polymerization of ε-caprolactone. Inorg. Chem. 2007, 46, 6565–6574. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Chan, C.Y.; Huang, C.A.; Chen, M.T.; Peng, K.F. Zinc anilido-oxazolinate complexes as initiators for ring opening polymerization. Dalton Trans. 2007, 4073–4078. [Google Scholar] [CrossRef]
- Chen, M.T.; Chen, C.T. Structural and catalytic studies of zinc complexes containing amido-oxazolinate ligands. Dalton Trans. 2011, 40, 12886–12894. [Google Scholar] [CrossRef] [PubMed]
- Dove, A.P.; Gibson, V.C.; Marshall, E.L.; White, A.J. P.; Williams, D.J. Magnesium and zinc complexes of a potentially tridentate β-diketiminate ligand. Dalton Trans. 2004, 570–578. [Google Scholar] [CrossRef]
- Abbina, S.; Du, G. Chiral amido-oxazolinate zinc complexes for asymmetric alternating copolymerization of CO2 and cyclohexene oxide. Organometallics 2012, 31, 7394–7403. [Google Scholar] [CrossRef]
- Dickson, R.S.; Fallon, G.D.; Zhang, Q.Q. Dimeric diphenylzinc adducts with cyclic thioethers. J. Chem. Soc. Dalton Trans. 2000, 1973–1974. [Google Scholar] [CrossRef]
- Li, C.Y.; Tsai, C.Y.; Lin, C.H.; Ko, B.T. Synthesis, structural characterization and reactivity of aluminium complexes supported by benzotriazole phenoxide ligands: Air-stable alumoxane as an efficient catalyst for ring-opening polymerization of l-lactide. Dalton Trans. 2011, 40, 1880–1887. [Google Scholar] [CrossRef] [PubMed]
- Bochmann, M.; Bwembya, G.; Webb, K.J.; Malik, M.A.; Walsh, J.R.; O’Brien, P. Arene chalcogenolato complexes of zinc and cadmium. Inorg. Synth. 1997, 31, 19–24. [Google Scholar]
- Sabatié, A.; Végh, D.; Loupy, A.; Floch, L. Synthesis of aromatic and heteroaromatic annelated [1,4]diazepines. ARKIVOC 2001, 6, 122–128. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXTL-97, Program for Refinement of Crystal Structures; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.-T.; Wang, M.-C.; Huang, T.-L. Zinc Complexes Containing Coumarin-Derived Anilido-Aldimine Ligands as Catalysts for Ring Opening Polymerization of L-Lactide. Molecules 2015, 20, 5313-5328. https://doi.org/10.3390/molecules20045313
Chen C-T, Wang M-C, Huang T-L. Zinc Complexes Containing Coumarin-Derived Anilido-Aldimine Ligands as Catalysts for Ring Opening Polymerization of L-Lactide. Molecules. 2015; 20(4):5313-5328. https://doi.org/10.3390/molecules20045313
Chicago/Turabian StyleChen, Chi-Tien, Min-Chian Wang, and Tzu-Lun Huang. 2015. "Zinc Complexes Containing Coumarin-Derived Anilido-Aldimine Ligands as Catalysts for Ring Opening Polymerization of L-Lactide" Molecules 20, no. 4: 5313-5328. https://doi.org/10.3390/molecules20045313
APA StyleChen, C. -T., Wang, M. -C., & Huang, T. -L. (2015). Zinc Complexes Containing Coumarin-Derived Anilido-Aldimine Ligands as Catalysts for Ring Opening Polymerization of L-Lactide. Molecules, 20(4), 5313-5328. https://doi.org/10.3390/molecules20045313