Synthesis, Structure and Antioxidant Activity of Cyclohexene-Fused Selenuranes and Related Derivatives
Abstract
:1. Introduction


2. Results and Discussion
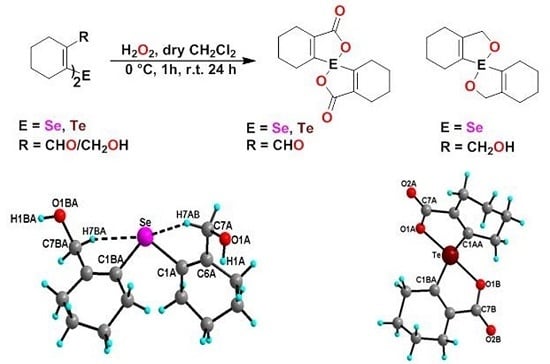
2.1. Synthesis

2.2. Spectroscopy Studies
2.3. X-ray Crystallographic Studies
2.3.1. Molecular Structure of 19



2.3.2. Molecular Structure of 20


2.3.3. Molecular Structure of 21


2.4. Glutathione Peroxidase-Like Activity
| Compound * | Average (V0) μM min−1 |
|---|---|
| Control | 10.5 ± 1.06 |
| Ebselen [47] | 27.8 ± 1.99 |
| Bis(o-formylphenyl)diselenide [24] | 34.3 ± 1.91 |
| 16 | 49.8 ± 1.61 |
| 17 | 9.4 ± 0.51 |
| 19 | 6.8 ± 0.77 |
| 20 | 16.4 ± 2.6 |
| 22 | 8.4 ± 0.24 |
2.4.1. Mechanistic Studies on Di-(2-formylcyclohexenyl)diselenide 16
2.4.2. Reaction of 16 with PhSH Followed by TBHP
2.4.3. Reaction of Di-(2-formylcyclohexenyl)diselenide 16 with TBHP Followed by PhSH


3. Experimental Section
3.1. General Information
| Compound | 19 | 20 | 21 |
|---|---|---|---|
| Empirical formula | C14H22O2Se | C56H64O16Se4 | C14H16O4Te |
| Formula weight | 301.28 | 1308.91 | 375.87 |
| Crystal system | Monoclinic | Monoclinic | Orthorhombic |
| Space group | P21/c | C2/c | Pbca |
| a (Å) | 8.7358(12) | 15.0340(13) | 15.7046(2) |
| b (Å) | 9.1761(12) | 8.5925(7) | 8.83038(16) |
| c (Å) | 19.689(2) | 10.4102(8) | 19.1603(3) |
| α (°) | 90 | 90 | 90 |
| β (°) | 116.096(8) | 112.358(4)) | 90 |
| γ (°) | 90 | 90 | 90 |
| V (Å3) | 1417.4(3) | 1242.69(18) | 2657.11(8) |
| Z | 4 | 1 | 8 |
| D (calcd) (Mg/m3) | 1.412 | 1.748 | 1.879 |
| Absorption coefficient (mm−1) | 2.639 | 2.027 | 17.759 |
| Reflections collected | 7290 | 11304 | 5111 |
| Final R(F) [I > 2σ(I)] a | 0.0567 | 0.0237 | 0.0515 |
| wR(F2) indices [I > 2σ(I)] | 0.1414 | 0.0597 | 0.1361 |
| Data/restraints/parameters | 2522/17/158 | 1963/0/87 | 2513/72/210 |
| Goodness-of-fit on F2 | 1.024 | 1.099 | 1.032 |
3.2. Synthesis




3.3. Coupled Reductase Assay

4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Epp, O.; Ladenstein, R.; Wendel, A. The refined structure of the selenoenzyme glutathione peroxidase at 0.2-nm resolution. Eur. J. Biochem. 1983, 133, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.R.; Beckett, G.J. Roles of selenium in type I iodothyronine 5′-deiodinase and in thyroid hormone and iodine metabolism. In Selenium in Biology and Human Health; Burk, R.F., Ed.; Springer-Verlag: New York, NY, USA, 1994. [Google Scholar]
- Berry, M.J.; Banu, L.; Larsen, P.R. Type I iodothyronine deiodinase is a selenocysteine-containing enzyme. Nature 1991, 349, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Behne, D.; Kyriakopoulos, A.; Meinhold, H.; Köhrle, J. Identification of type I iodothyronine 5′-deiodinase as a selenoenzyme. Biochem. Biophys. Res. Commun. 1990, 173, 1143–1149. [Google Scholar] [CrossRef]
- Bianco, A.C.; Salvatore, D.; Gereben, B.; Berry, M.J.; Larsen, P.R. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr. Rev. 2002, 23, 38–89. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-R.; Kim, J.-R.; Kwon, K.-S.; Yoon, H.W.; Levine, R.L.; Ginsburg, A.; Rhee, S.G. Molecular cloning and characterization of a mitochondrial selenocysteine-containing thioredoxin reductase from rat liver. J. Biol. Chem. 1999, 274, 4722–4734. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.H., Jr.; Arscott, L.D.; Müller, S.; Lennon, B.W.; Ludwig, M.L.; Wang, P.F.; Veine, D.M.; Becker, K.; Schirmer, R.H. Thioredoxin reductase two modes of catalysis have evolved. Eur. J. Biochem. 2000, 267, 6110–6117. [Google Scholar] [CrossRef] [PubMed]
- Mugesh, G.; Singh, H.B. Synthetic organoselenium compounds as antioxidants: Glutathione peroxidiseactivity. Chem. Soc. Rev. 2000, 29, 347–357. [Google Scholar] [CrossRef]
- Mugesh, G.; du Mont, W.W.; Sies, H. Chemistry of biologically important synthetic organoseleniumcompounds. Chem. Rev. 2001, 101, 2125–2179. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Rocha, J.B.T. Organoselenium and organotellurium compounds: Toxicology and pharmacology. In Organic Selenium and Tellurium Compounds; Rappoport, Z., Ed.; Wiley: Chichester, UK, 2012; Volume 3, Part II, Chapter 21. [Google Scholar]
- Masukawa, T. Pharmacological and toxicological aspects of inorganic and organic selenium compounds. In The Chemistry of Organoselenium and Tellurium Compounds; Patai, S., Ed.; Wiley: Chichester, UK, 1987; Volume 2, Chapter 9. [Google Scholar]
- Jamier, V.; Ba, L.A.; Jacob, C. Selenium- and tellurium-containing multifunctional redox agents as biochemical redox modulators with selective cytotoxicity. Chem. Eur. J. 2010, 16, 10920–10928. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, D.; Mugesh, G. Enzyme mimetic chemistry of organoselenium compounds. In The Chemistry of Organoselenium and Tellurium Compounds; Patai, S., Ed.; Wiley: Chichester, UK, 2013; Volume 4, Part II, Chapter 16. [Google Scholar]
- Bhabak, K.P.; Mugesh, G. Functional mimics of glutathione peroxidase: Bioinspired synthetic antioxidant. Acc. Chem. Res. 2010, 43, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, B.J.; Lamani, D.S.; Mugesh, G.; Wirth, T. Current research on mimics and models of selenium-containing antioxidants. In Handbook of Chalcogen Chemistry: New Perspectives in Sulfur, Selenium and Tellurium, 2nd ed.; RSC: Cambridge, UK, 2013. [Google Scholar]
- Mugesh, G. Glutathione peroxidase activity of ebselen and its analogues: Some insights into the complex chemical mechanisms underlying the antioxidant activity. Curr. Chem. Biol. 2013, 7, 47–56. [Google Scholar] [CrossRef]
- Mugesh, G.; Panda, A.; Singh, H.B.; Punekar, N.S.; Butcher, R.J. Glutathione peroxidase-like antioxidant Activity of diaryl diselenides: A mechanistic study. J. Am. Chem. Soc. 2001, 123, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.J.; Zade, S.S.; Singh, H.B.; Sunoj, R.B. Organoselenium chemistry: Role of Intramolecular interactions. Chem. Rev. 2010, 110, 4357–4416. [Google Scholar] [CrossRef] [PubMed]
- Bayse, C.A.; Pavlou, A. Tuning the activity of glutathione peroxidase mimics through intramolecular Se···N,O interactions: A DFT study incorporating solvent-assisted proton exchange (SAPE). Org. Biomol. Chem. 2011, 9, 8006–8015. [Google Scholar] [CrossRef] [PubMed]
- Back, T.G.; Moussa, Z.; Parvez, M. The exceptional glutathione peroxidase-like activity of di(3-hydroxypropyl) selenide and the unexpected role of a novel spirodioxaselenanonane intermediate in the catalytic cycle. Angew. Chem. Int. Ed. 2004, 43, 1268–1270. [Google Scholar] [CrossRef] [PubMed]
- Back, T.G.; Moussa, Z. Remarkable activity of a novel cyclic seleninate ester as a glutathione peroxidase mimetic and its facile in situ generation from allyl 3-hydroxypropyl selenide. J. Am. Chem. Soc. 2002, 124, 12104–12105. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.K.; Patel, U.; Roy, D.; Sunoj, R.B.; Singh, H.B.; Wolmershäuser, G.; Butcher, R.J. o-Hydroxylmethylphenylchalcogens: Synthesis, intramolecular nonbonded chalcogen···OH interactions, and glutathione peroxidase-like activity. J. Org. Chem. 2005, 70, 9237–9247. [Google Scholar] [CrossRef] [PubMed]
- Back, T.G.; Kuzma, D.; Parvez, M. Aromatic derivatives and tellurium analogues of cyclic seleninate esters and spirodioxyselenuranes that act as glutathione peroxidase mimetics. J. Org. Chem. 2005, 70, 9230–9236. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Panda, S.; Singh, H.; Wolmershäuser, G.; Butcher, R. Structural aspects of some organoselenium compounds. Struct. Chem. 2007, 18, 127–132. [Google Scholar] [CrossRef]
- Press, D.J.; Mercier, E.A.; Kuzma, D.A.; Back, T.G. Substituent effects upon the catalytic activity of aromatic cyclic seleninate esters and spirodioxyselenuranes that act as glutathione peroxidase mimetics. J. Org. Chem. 2008, 73, 4252–4255. [Google Scholar] [CrossRef] [PubMed]
- Back, T.G.; Moussa, Z.J. Diselenides and allyl selenides as glutathione peroxidase mimetics. Remarkable activity of cyclic seleninates produced in situ by the oxidation of allyl ö-hydroxyalkyl selenides. J. Am. Chem. Soc. 2003, 125, 13455–13460. [Google Scholar] [CrossRef] [PubMed]
- Press, D.J.; McNeil, N.M.R.; Hambrook, M.; Back, T.G. Effects of methoxy substituents on the glutathione peroxidase-like activity of cyclic seleninate esters. J. Org. Chem. 2014, 79, 9394–9401. [Google Scholar] [CrossRef] [PubMed]
- Kuzma, D.; Parvez, M.; Back, T.G. Formation of a spirodiazaselenurane and its corresponding azaselenonium derivatives from the oxidation of 2,2′-selenobis(benzamide). Structure, properties and glutathione peroxidase activity. Org. Biomol. Chem. 2007, 5, 3213–3217. [Google Scholar] [CrossRef] [PubMed]
- Sarma, B.K.; Manna, D.; Minoura, M.; Mugesh, G. Synthesis, structure, spirocyclization mechanism, and glutathione peroxidase-like antioxidant activity of stable spirodiazaselenurane and spirodiazatellurane. J. Am. Chem. Soc. 2010, 132, 5364–5374. [Google Scholar] [CrossRef] [PubMed]
- Lamani, D.S.; Bhowmick, D.; Mugesh, G. Spirodiazaselenuranes: Synthesis, structure and antioxidant activity. Org. Biomol. Chem. 2012, 10, 7933–7943. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, K.; Singh, H.B.; Goel, N.; Singh, U.P.; Butcher, R.J. Synthesis and structural characterization of pincer type bicyclic diacyloxy- and diazaselenuranes. Dalton Trans. 2011, 40, 9858–9867. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.R.; Singh, H.B.; Butcher, R.J. Synthesis, structure and reactivity of β-chalcocyclohexenals: dichalcogenides and chalcogenides. Dalton Trans. 2015. (submitted). [Google Scholar]
- Minkin, V.I.; Sadekov, I.D.; Rivkin, B.B.; Zakharov, A.V.; Nivorozhkin, V.L.; Kompan, O.E.; Struchkov, Y.T. Synthesis and structure of β-tellurovinylcarbonyl compounds. J. Organomet. Chem. 1997, 536, 233–248. [Google Scholar] [CrossRef]
- Takaguchi, Y.; Furukawa, N. First synthesis and structural determination of 1,1′-spirobis(3H-2, 1-benzoxatellurole)-3,3′-dione ([10-Te-4(C202)]). Heteroat. Chem. 1995, 6, 481–485. [Google Scholar] [CrossRef]
- Press, D.J.; McNeil, N.M.R.; Rauk, A.; Back, T.G. NMR and Computational Studies of the configurational properties of spirodioxyselenuranes. Are dynamic exchange processes or temperature-dependent chemical shifts involved? J. Org.Chem. 2012, 77, 9268–9276. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Menon, S.C.; Singh, H.B.; Butcher, R.J. Synthesis of some macrocycles:Bicycles from bis(o-formylphenyl) selenide: X-ray crystal structure of bis(o-formylphenyl) selenide and the first 28-membered selenium containing macrocyclic ligand. J. Organomet. Chem. 2001, 623, 87–94. [Google Scholar] [CrossRef]
- Iwaoka, M.; Tomoda, S. First observation of a C-H...Se “hydrogen bond”. J. Am. Chem. Soc. 1994, 116, 4463–4464. [Google Scholar] [CrossRef]
- Cordero, B.; Gomez, V.; Platero-Prats, A.E.; Reves, M.; Echeverria, J.; Cremades, E.; Barragan, F.; Alvarez, S. Covalent radii revisited. Dalton Trans. 2008, 21, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Bader, R.F.W. Atoms in Molecules: A Quantum Theory; Oxford University Press: New York, NY, USA, 1990. [Google Scholar]
- Popelier, P. Atoms in molecules: An introduction; Pearson: Harlow, UK, 2000. [Google Scholar]
- Matta, C.F.; Boyd, R.J. The Quantum Theory of Atoms in Molecules; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Biegler-Konig, F.; Schonbohm, J.; Bayles, D. Software news and updates AIM2000-a program to analyze and visualize atoms in molecules. J. Comput. Chem. 2001, 22, 545–559. [Google Scholar]
- Dahlén, B.; Lindgren, B. Formatin and crystal structure of 3,3′-spirobi(3-selenaphthalide). Acta Chem. Scand. 1993, 27, 2218–2220. [Google Scholar] [CrossRef]
- Dahlen, B. The molecular structure of o-carboxyphenyl methyl sulphoxide and o-carboxyphenyl methyl selenium oxide. Acta Crystallogr. B 1973, 29, 595–602. [Google Scholar] [CrossRef]
- Wilson, S.R.; Zucker, P.A.; Huang, R.R.; Spector, A. Development of synthetic compounds with glotathione peroxidase activity. J. Am. Chem. Soc. 1989, 111, 5936–5939. [Google Scholar] [CrossRef]
- Müller, A.; Cadenas, E.; Graf, P.; Sies, H. A novel biologically active seleno-organic compound-1: Glutathione peroxidase-like activity in vitro and antioxidant capacity of PZ 51 (Ebselen). Biochem. Pharmacol. 1984, 33, 3235–3239. [Google Scholar] [CrossRef]
- Perrin, D.D.; Armargo, W.L.F.; Perrin, D.R. Purification of Laboratory Chemicals, 4th ed.; Pergamon Press: Oxford, UK, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXS-97, Program for Crystal Structure Solution University of Göttingen; University of Göttingen: Gottingen, Germany, 1990. [Google Scholar]
- Sheldrick, G.M. SHELXL-97, Program for Crystal Structure Refinement; University of Göttingen: Gottingen, Germany, 1997. [Google Scholar]
- Sample Availability: Samples of the compounds not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasad, P.R.; Singh, H.B.; Butcher, R.J. Synthesis, Structure and Antioxidant Activity of Cyclohexene-Fused Selenuranes and Related Derivatives. Molecules 2015, 20, 12670-12685. https://doi.org/10.3390/molecules200712670
Prasad PR, Singh HB, Butcher RJ. Synthesis, Structure and Antioxidant Activity of Cyclohexene-Fused Selenuranes and Related Derivatives. Molecules. 2015; 20(7):12670-12685. https://doi.org/10.3390/molecules200712670
Chicago/Turabian StylePrasad, Poonam Rajesh, Harkesh B. Singh, and Ray J. Butcher. 2015. "Synthesis, Structure and Antioxidant Activity of Cyclohexene-Fused Selenuranes and Related Derivatives" Molecules 20, no. 7: 12670-12685. https://doi.org/10.3390/molecules200712670
APA StylePrasad, P. R., Singh, H. B., & Butcher, R. J. (2015). Synthesis, Structure and Antioxidant Activity of Cyclohexene-Fused Selenuranes and Related Derivatives. Molecules, 20(7), 12670-12685. https://doi.org/10.3390/molecules200712670