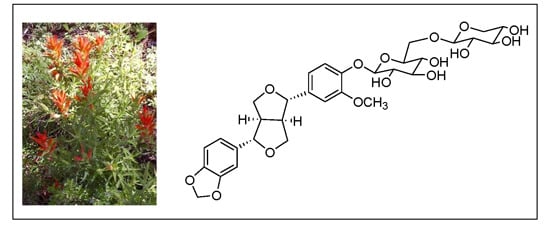
A New Furofuran Lignan Diglycoside and Other Secondary Metabolites from the Antidepressant Extract of Castilleja tenuiflora Benth
Abstract
:1. Introduction
2. Results and Discussion








| (1a) | Tenuifloroside (1) | ||||
|---|---|---|---|---|---|
| Position | δC, Type | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 135.1, C | - | 135.59 | - | |
| 2 | 110.5, CH | 6.93 (1H, d, 2.0) | 110.87 | 6.91 (1H, d, 1.6) | |
| 3 | 150.6, C | - | 149.24 | - | |
| 4 | 145.9, C | - | 146.20 | - | |
| 5 | 119.2, CH | 7.07 (1H, d, 8.4) | 115.88 | 7.07 (1H, d, 8.4) | |
| 6 | 118.7, CH | 6.90 (1H, dd, 8.4, 2.0) | 118.82 | 6.82 (1H, dd, 8.4, 1.6) | |
| 7 | 85.6, CH | 4.77 (1H, d, 4.8) | 85.40 | 4.6 (1H, d, 4.8) | |
| 8 | 54.2, CH | 3.02–3.06 (1H, m) | 54.13 | 2.97 (1H, m) | |
| 9 | 72.08, CH2 | 4.26 (1H, dd, 9.2, 6.8) | 71.45 | 4.13 (1H, m) | |
| 3.88 (1H, dd, 4.0, 4.0) | 3.74 (1H, dd, 4.0, 4.0) | ||||
| 1′ | 137.7, C | 135.91 | - | ||
| 2′ | 106.6, CH | 6.84 (1H, d, 1.56) | 107.00 | 6.89 (1H, d, 1.2) | |
| 3′ | 147.2, C | - | 146.91 | - | |
| 4′ | 148.1, C | - | 147.84 | - | |
| 5′ | 108.3, CH | 6.76 (1H, d, 8.0) | 108.47 | 6.83 (1H, d, 8.4) | |
| 6′ | 119.6, CH | 6.80 (1H, dd, 8.0, 1.56,) | 118.85 | 6.83 (1H, 8.4, 1.2) | |
| 7′ | 86.01, CH | 4.69 (1H, d, 5.2) | 85.30 | 4.6 (1H, d, 4.8) | |
| 8′ | 54.46, CH | 3.07–3.12 (1H, m) | 54.17 | 2.97 (1H, m) | |
| 9′ | 71.8, CH2 | 4.21 (1H, dd, 9.2, 6.8) | 71.45 | 4.13 (1H, m) | |
| 3.86 (1H, dd, 4.0, 4.0) | 3.74 (1H, dd, 4.0, 4.0) | ||||
| OCH3 | 56.28 | 3.83 (3H, s) | 56.14 | 3.74 (3H, s) | |
| OCH2O | 101.26 | 5.93 (2H, s) | 101.32 | 5.95 (2H, s) | |
| Glc | 1 | 100.8 CH | 4.92 (1H, d, 8.0) | 100.62 | 4.82 (1H, d, 7.6) |
| 2 | 72.6, CH | 5.25 (1H, dd, 8.0,9.6) | 73.59 | 3.24 (1H, m) | |
| 3 | 71.3, CH | 5.22 (1H, dd, 9.6,9.6) | 77.06 | 3.24 (1H, m) | |
| 4 | 68.8, CH | 4.99 (1H, dd, 9.6, 9.6) | 69.98 | 3.14 (1H, m) | |
| 5 | 73.97, CH | 3.74 (1H, ddd, 9.6, 6.6, 2.0) | 76.35 | 3.49 (1H, m) | |
| 6α | 67.60, CH2 | 3.80–3.85 (1H, m) | 68.47 | 3.90 (1H, d br, 10.4) | |
| 6β | 3.66 (1H, dd, 11.2, 6.6) | 3.53 (1H, dd, 11.6, 6.4) | |||
| Xyl | 1 | 100.53, CH | 4.53 (1H, d, 6.8) | 104.12 | 4.13(1H, m) |
| 2 | 70.66, CH | 4.87 (1H, dd, 8.4, 6.8) | 73.81 | 2.93 (1H, m) | |
| 3 | 71.29, CH | 5.09 (1H, dd, 8.4, 8.4) | 76.89 | 3.04 (1H, m) | |
| 4 | 68.96, CH | 4.91 (1H, ddd,8.4,8.4, 4.8) | 69.98 | 3.24 (1H, m) | |
| 5α | 62.11, CH2 | 4.09 (1H, dd, 11.6, 8.8) | 65.98 | 2.90 (1H, m) | |
| 5β | 3.26 (1H, dd, 11.6, 4.8) | 3.64 (1H, dd, 5.6, 11.2) | |||
3. Experimental Section
3.1. General Information
3.2. Plant Materials
3.3. Extraction and Isolation of Chemicals Compounds from MeOH Extract
3.4. Acetylation of Compound 1
3.5. HPLC Analysis
3.6. Bioactivity Assays
3.6.1. Animals
3.6.2. Drugs
3.6.3. Experimental Design
3.6.4. Sedative Effect
Pentobarbital-Induced Hypnosis (Pbi)
3.6.5. Elevated Plus Maze (EPM)
3.6.6. Open Field Test (OFT)
3.6.7. Forced Swimming Test (FST)
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Holmgren, N.H. Four new species of mexican castilleja (subgenus Castilleja, Scrophulariaceae) and their relatives. Brittonia 1976, 28, 195–208. [Google Scholar] [CrossRef]
- Graham, J.G.; Quinn, M.L.; Fabricant, D.S.; Farnsworth, N.R. Plants used against cancer-an extension of the work of Jonathan Hartwell. J. Ethnopharmacol. 2000, 73, 347–377. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J.; Villarreal, M.L.; Salazar-Olivo, L.A.; Gomez-Sanchez, M.; Dominguez, F.; Garcia-Carranca, A. Mexican medicinal plants used for cancer treatment: Pharmacological, phytochemical and ethnobotanical studies. J. Ethnopharmacol. 2011, 133, 945–972. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M. Catálogo de Nombres Vulgares y Científicos de Plantas Mexicanas, 1st ed.; Fondo de Cultura Económica: México, D.F., Mexico, 1979; p. 1038. [Google Scholar]
- Argueta Villamar, A.; Cano Asseleh, L.M.; Rodarte, M.E. Atlas de las Plantas de la Medicina Tradicional Mexicana; Instituto Nacional Indigenista: México, D.F., Mexico, 1994; Volume 2, pp. 662–663. [Google Scholar]
- Sanchez, P.M.; Villarreal, M.L.; Herrera-Ruiz, M.; Zamilpa, A.; Jiménez-Ferrer, E.; Trejo-Tapia, G. In vivo anti-inflammatory and anti-ulcerogenic activities of extracts from wild growing and in vitro plants of Castilleja tenuiflora Benth. (Orobanchaceae). J. Ethnopharmacol. 2013, 150, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Escobar, J.A.; Bazaldúa, S.; Villarreal, M.L.; Bonilla-Barbosa, J.R.; Mendoza, S.; Rodríguez-López, V. Cytotoxic and antioxidant activities of selected Lamiales species from Mexico. Pharm. Biol. 2011, 49, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Ocampo, D.; Bazaldúa-Gómez, S.; Bonilla-Barbosa, J.R.; Aburto-Amar, R.; Rodríguez-López, V. Anti-inflammatory activity of iridoids and verbascoside isolated from Castilleja tenuiflora. Molecules 2013, 18, 12109–12118. [Google Scholar] [CrossRef] [PubMed]
- Béjar, E.; Reyes-Chilpa, R.; Jiménez-Estrada, M. Bioactive Natural Products (Part E). Stud. Nat. Prod. Chem. 2000, 24, 799–844. [Google Scholar]
- Gómez-Aguirre, Y.A.; Zamilpa, A.; González-Cortazar, M.; Trejo-Tapia, G. Adventitious root cultures of Castilleja tenuiflora Benth. as a source of phenylethanoid glycosides. Ind. Crops Prod. 2012, 36, 188–195. [Google Scholar] [CrossRef]
- López-Laredo, A.R.; Gómez-Aguirre, Y.A.; Medina-Pérez, V.; Salcedo-Morales, G.; Sepúlveda-Jiménez, G.; Trejo-Tapia, G. Variation in antioxidant properties and phenolics concentration in different organs of wild growing and greenhouse cultivated Castilleja tenuiflora Benth. Acta Physiol. Plant. 2012, 34, 2435–2442. [Google Scholar] [CrossRef]
- Vogel, H.G. Psychotropic and Neurotropic Activity. In Drug Discovery and Evaluation: Pharmacological Assays, 3rd ed.; Springer-Verlag: München, DE, Germany, 2008; pp. 565–875. [Google Scholar]
- Nogueira, E.; Vassilieff, V.S. Hypnotic, anticonvulsant and muscle relaxant effects of Rubus brasiliensis. Involvement of GABA(A)-system. J. Ethnopharmacol. 2000, 70, 275–280. [Google Scholar] [CrossRef]
- Akanmu, M.A.; Honda, K.; Inoué, S. Hypnotic effects of total aqueous extracts of Vervain hastata (Verbenaceae) in rats. Psych. Clin. Neurosci. 2002, 56, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Koo, K.A.; Kim, S.H.; Oh, T.H.; Kim, Y.C. Acteoside and its aglycones protect primary cultures of rat cortical cells from glutamate-induced excitotoxicity. Life Sci. 2006, 79, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Lei, W.; Liu, X.; Yan, S.; Yan, S.S.; Wang, Y.; Zhang, W. Multi-faced neuroprotective effects of geniposide depending on the RAGE-mediated signaling in an Alzheimer mouse model. Neuropharmacology 2012, 89, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Losi, G.; Puia, G.; Garzon, G.; De Vuono, M.C.; Baraldi, M. Apigenin modulates GABAergic and glutamatergic transmission in cultured cortical neurons. Eur. J. Pharmacol. 2004, 502, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.Y.; Lu, Y.N.; Fang, X.M.; Xu, Y.P.; Gao, G.Z.; Jin, L.J. Neuroprotective properties of aucubin in diabetic rats and diabetic encephalopathy rats. Mol. Biol. Rep. 2012, 39, 9311–9318. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, C.S.; Hu, Z.; Han, J.Y.; Kim, S.K.; Yoo, S.K.; Yeo, Y.M.; Chong, M.S.; Lee, K.; Hong, J.T.; et al. Enhancement of pentobarbital-induced sleep by apigenin through cloride ion channel activation. Arch. Pharm. Res. 2012, 35, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Masur, J.; Märtz, R.M.W.; Carlini, E.A. Effects of acute and chronic administration of Cannabis sativa and (−)Δ9-trans-tetrahydrocannabinol on the behavior of rats in an open-field arena. Psychopharmacology 1971, 19, 388–397. [Google Scholar] [CrossRef]
- Julião, L. de S.; Leitão, S.G.; Lotti, C.; Picinelli, A.L.; Rastrelli, L.; Fernandes, P.D.; Noël, F.; Thibaut, J.P.B.; Leitão, G.G. Flavones and phenylpropanoids from a sedative extract of Lantana trifolia L. Phytochemistry 2010, 71, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Petit-Demouliere, B.; Chenu, F.; Bourin, M. Forced swimming test in mice: A review of antidepressant activity. Psychopharmacology 2005, 177, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, I.A.; Dar, A. Behavioral profile of Hypericum perforatum (St. John’s Wort) extract. A comparison with standard antidepressants in animal models of depression. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1082–1089. [Google Scholar] [PubMed]
- Zaman, A.; Sultan Khan, M.S.; Akter, L.; Syeed, S.H.; Akter, J.; Mamun, A.A.; Alam, M.E.; Habib, M.A.; Jalil, M.A. Exploring new pharmacology and toxicological screening and safety evaluation of one widely used formulation of Nidrakar Bati from South Asia region. BMC Complement. Altern. Med. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Kebebew, Z.; Shibeshi, W. Evaluation of anxiolytic and sedative effects of 80% ethanolic Carica papaya L. (Caricaceae) pulp extract in mice. J. Ethnopharmacol. 2013, 150, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Ambawade, S.; Kasture, V.S.; Kasture, S.B. Anxiolytic activity of Glycyrrhiza glabra Linn. J. Nat. Rem. 2001, 1, 130–134. [Google Scholar]
- Ambavade, S.D.; Mhetre, N.A.; Muthal, A.P.; Bodhankar, S.L. Pharmacological evaluation of anticonvulsant activity of root extract of Saussurea lappa in mice. Eur. J. Integr. Med. 2009, 1, 131–137. [Google Scholar] [CrossRef]
- Calabrese, E.J. Biphasic dose responses in biology, toxicology and medicine: Accounting for their generalizability and quantitative features. Environ. Pollut. 2013, 182, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Angelis, L. Experimental anxiety and antidepressant drugs: The effects of moclobemide, a selective reversible MAO-A inhibitor, fluoxetine and imipramine in mice. Naunyn-Schmiedebergs Arch. Pharmacol. 1996, 354, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.C.; Lin, M.C.; Chen, G.F.; Yiu, T.J.; Tzen, J.T.C. Identification of methanol-soluble compounds in sesame and evaluation of antioxidant potential of its lignans. J. Agric. Food Chem. 2011, 59, 3214–3219. [Google Scholar] [CrossRef] [PubMed]
- Ogiku, T.; Yoshida, S.; Ohmizu, H.; Iwaasaki, T. Stereocontrolled Syntheses of Diequatorial and Axial-Equatorial Furofuran Lignans. J. Org. Chem. 1995, 60, 1148–1153. [Google Scholar] [CrossRef]
- Grougnet, R.; Magiatis, P.; Laborie, H.; Lazarou, D.; Papadopoulos, A.; Skaltsounis, A.L. Sesamolinol glucoside, disaminyl ether, and other lignans from sesame seeds. J. Agric. Food. Chem. 2012, 60, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.R.; Moon, H.T.; Lee, D.G.; Woo, E.R. A new lignan glycoside from the stem bark of Styrax japonica S. et Z. Arch. Pharm. Res. 2007, 30, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Katayama, T.; Davin, L.B.; Lewis, N.G. An extraordinary accumulation of (−)-pinoresinol in cell-free extracts of Forsythia intermedia: Evidence for enantiospecific reduction of (+)-pinoresinol. Phytochemistry 1992, 31, 3875–3881. [Google Scholar] [CrossRef]
- Damtoft, S. Iridoid glucosides from Lamium album. Phytochemistry 1992, 31, 175–178. [Google Scholar] [CrossRef]
- Zhang, Z.J. Therapeutic effects of herbal extracts and constituents in animal models of psychiatric disorders. Life Sci. 2004, 75, 1659–1699. [Google Scholar] [CrossRef] [PubMed]
- Pellow, S.; File, S.E. Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: A novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 1986, 24, 525–529. [Google Scholar] [CrossRef]
- Lister, R.G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology 1987, 92, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of tenuifloroside, verbascoside, apigenin, luteolin 5-methyl ether and mixure of geniposide and caryoptoside are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Ruiz, M.; López-Rodríguez, R.; Trejo-Tapia, G.; Domínguez-Mendoza, B.E.; González-Cortazar, M.; Tortoriello, J.; Zamilpa, A. A New Furofuran Lignan Diglycoside and Other Secondary Metabolites from the Antidepressant Extract of Castilleja tenuiflora Benth. Molecules 2015, 20, 13127-13143. https://doi.org/10.3390/molecules200713127
Herrera-Ruiz M, López-Rodríguez R, Trejo-Tapia G, Domínguez-Mendoza BE, González-Cortazar M, Tortoriello J, Zamilpa A. A New Furofuran Lignan Diglycoside and Other Secondary Metabolites from the Antidepressant Extract of Castilleja tenuiflora Benth. Molecules. 2015; 20(7):13127-13143. https://doi.org/10.3390/molecules200713127
Chicago/Turabian StyleHerrera-Ruiz, Maribel, Ricardo López-Rodríguez, Gabriela Trejo-Tapia, Blanca Eda Domínguez-Mendoza, Manases González-Cortazar, Jaime Tortoriello, and Alejandro Zamilpa. 2015. "A New Furofuran Lignan Diglycoside and Other Secondary Metabolites from the Antidepressant Extract of Castilleja tenuiflora Benth" Molecules 20, no. 7: 13127-13143. https://doi.org/10.3390/molecules200713127
APA StyleHerrera-Ruiz, M., López-Rodríguez, R., Trejo-Tapia, G., Domínguez-Mendoza, B. E., González-Cortazar, M., Tortoriello, J., & Zamilpa, A. (2015). A New Furofuran Lignan Diglycoside and Other Secondary Metabolites from the Antidepressant Extract of Castilleja tenuiflora Benth. Molecules, 20(7), 13127-13143. https://doi.org/10.3390/molecules200713127