Involvement of the Heme-Oxygenase Pathway in the Antiallodynic and Antihyperalgesic Activity of Harpagophytum procumbens in Rats
Abstract
:1. Introduction
2. Results and Discussion
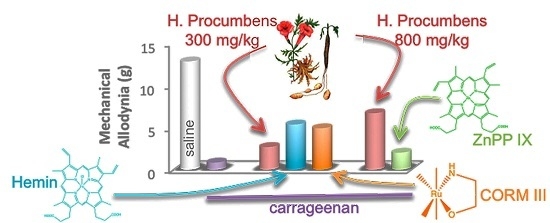
2.1. Mechanical Allodynia




2.2. Thermal Hyperalgesia
3. Experimental Section
3.1. Animals
3.2. Carrageenan-Induced Inflammatory Pain in Rats
3.3. Mechanical Allodynia
3.4. Thermal Hyperalgesia
3.5. Experimental Design
3.6. Drugs
Plant Extract
3.7. Statistical Analysis
Author Contributions
Conflicts of Interest
References
- Kidd, B.L.; Urban, L.A. Mechanisms of inflammatory pain. Br. J. Anaesth. 2001, 87, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef] [PubMed]
- Parenti, C.; Marrazzo, A.; Aricò, G.; Parenti, R.; Pasquinucci, L.; Ronsisvalle, S.; Ronsisvalle, G.; Scoto, G.M. The antagonistic effect of the sigma 1 receptor ligand (+)-MR200 on persistent pain induced by inflammation. Inflamm. Res. 2014, 63, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Linley, J.E.; Rose, K.; Ooil, L.; Gamper, M. Understanding inflammatory pain: Ion channels contributing to acute and chronic nociception. Pflüg. Arch. -Eur. J. Physiol. 2010, 459, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Ussai, S.; Miceli, L.; Pisa, F.E.; Bednarova, R.; Giordano, A.; Della Rocca, G.; Petelin, R. Impact of potential inappropriate NSAIDs use in chronic pain. Drug Des. Dev. Ther. 2015, 9, 2073–2077. [Google Scholar] [CrossRef] [PubMed]
- Quintans, J.S.; Antoniolli, A.R.; Almeida, J.R.; Santana-Filho, V.J.; Quintans-Júnior, L.J. Natural products evaluated in neuropathic pain models—A systematic review. Basic Clin. Pharmacol. Toxicol. 2014, 114, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Mncwangi, N.; Chen, W.; Vermaak, I.; Viljoen, A.M.; Gericke, N. Devil’s Claw-a review of the ethnobotany, phytochemistry and biological activity of Harpagophytum procumbens. J. Ethnopharmacol. 2012, 143, 755–771. [Google Scholar] [CrossRef] [PubMed]
- Grant, L.; McBean, D.E.; Fyfe, L.; Warnock, A.M. A review of the biological and potential therapeutic actions of Harpagophytum procumbens. Phytother. Res. 2007, 21, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.W.; Kim, J.G.; Han, D.; Kim, Y.T. Analgesic effect of Harpagophytum procumbens on postoperative and neuropathic pain in rats. Molecules 2014, 19, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.L.; Santos, E.H.R.; Seabra, M.; de Lourdes, V.; da Silva, A.A.B.; Tufik, S. Evaluation of acute and chronic treatments with Harpagophytum procumbens on Freund’s adjuvant-induced arthritis in rats. J. Ethnopharmacol. 2004, 91, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Lanhers, M.C.; Fleurentin, J.; Mortier, F.; Vinche, A.; Younos, C. Anti-inflammatory and analgesic effects of an aqueous extract of Harpagophytum procumbens. Planta Med. 1992, 58, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Steiner, A.A.; Branco, L.G.; Cunha, F.Q.; Ferreira, S.H. Role of the haemeoxygenase/carbon monoxide pathway in mechanical nociceptor hypersensitivity. Br. J. Pharmacol. 2001, 132, 1673–1682. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, M.J.; Fernández, P.; Guillén, M.I. Anti-inflammatoryactions of the hemeoxygenase-1 pathway. Curr. Pharm. Des. 2003, 9, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H.M.; Song, W. A heme oxygenase-1 transducer model of degenerative and developmental brain disorders. Int. J. Mol. Sci. 2015, 16, 5400–5419. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.G.; Branco, L.G. Role of the peripheral heme oxygenase-carbon monoxide pathway on the nociceptive response of rats to the formalin test: Evidence for a cGMP signaling pathway. Eur. J. Pharmacol. 2007, 556, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, C.G.; Branco, L.G. Role of the spinal cord heme oxygenase-carbon monoxide-cGMP pathway in the nociceptive response of rats. Eur. J. Pharmacol. 2008, 581, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.O.; Egea, J.; Lorrio, S.; Rojo, A.I.; Cuadrado, A.; López, M.G. Nrf2-mediated heme oxygenase-1 up-regulation induced by cobalt protoporphyrin has antinociceptive effects against inflammatory pain in the formalin test in mice. Pain 2008, 137, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Egea, J.; Rosa, A.O.; Lorrio, S.; del Barrio, L.; Cuadrado, A.; López, M.G. Haeme oxygenase-1 overexpression via nAChRs and the transcription factor Nrf2 has antinociceptive effects in the formalin test. Pain 2009, 146, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Olszanecki, R.; Gryglewski, R.; Schwartzman, M.L.; Lianos, E.; Kappas, A.; Nasjletti, A.; Abraham, N.G. Regulation of cyclooxygenase by the heme-heme oxygenase system in microvessel endothelial cells. J. Pharmacol. Exp. Ther. 2002, 300, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Grangeiro, N.M.; Aguiar, J.A.; Chaves, H.V.; Silva, A.A.; Lima, V.; Benevides, N.M.; Brito, G.A.; da Graça, J.R.; Bezerra, M.M. Heme oxygenase/carbon monoxide-biliverdin pathway may be involved in the antinociceptive activity of etoricoxib, a selective COX-2inhibitor. Pharmacol. Rep. 2011, 63, 112–119. [Google Scholar] [CrossRef]
- Lee, D.S.; Choi, H.G.; Wan Woo, K.; Kang, D.G.; Lee, H.S.; Oh, H.; Ro Lee, K.; Kim, Y.C. Pulchellamin G, an amino acid-sesquiterpene lactone, from Saussureapulchella suppresses lipopolysaccharide-induced inflammatory responses via heme oxygenase-1expression in murine peritoneal macrophages. Eur. J. Pharmacol. 2013, 715, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Carcolé, M.; Castany, S.; Leánez, S.; Pol, O. Treatment with a hemeoxygenase 1 inducer enhances the antinociceptive effects of µ-opioid, δ-opioid, and cannabinoid 2 receptors during inflammatory pain. J. Pharmacol. Exp. Ther. 2014, 351, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.H.; Lim, S.; Han, S.M.; Park, H.J.; Shin, I.; Kim, J.W.; Kim, N.J.; Lee, J.S.; Kim, K.A.; Kim, C.J. Harpagophytum procumbens suppresses lipopolysaccharide-stimulated expressions of cyclooxygenase-2 and inducible nitric oxide synthase in fibroblast cell line L929. J. Pharmacol. Sci. 2003, 93, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Na, H.-K.; Mossanda, K.S.; Lee, J.-Y.; Surh, Y.-J. Inhibition of phorbol ester-induced COX-2 expression by some edible African plants. Biofactors 2004, 21, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.K.; Mossanda, K.S.; Na, H.-K.; Surh, Y.-J. Inhibitory effects of the extracts of Sutherlandia frutescens (L.) R. Br. and Harpagophytum procumbens DC. On phorbol ester-induced COX-2 expression in mouse skin: AP-1 and CREB as potential upstream targets. Cancer Lett. 2005, 218, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Choy, C.S.; Hu, C.M.; Chiu, W.T.; Lam, C.S.; Ting, Y.; Tsai, S.H.; Wang, T.C. Suppression of lipopolysaccharide-induced of inducible nitric oxide synthase and cyclooxygenase-2 by Sanguis Draconis, a dragon’s blood resin, in RAW 264.7 cells. J. Ethnopharmacol. 2008, 115, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Hervera, A.; Leánez, S.; Negrete, R.; Motterlini, R.; Pol, O. Carbon monoxide reduces neuropathic pain and spinal microglial activation by inhibiting nitric oxide synthesis in mice. PLoS ONE 2012, 7, e43693. [Google Scholar] [CrossRef] [PubMed]
- Parenti, C.; Turnaturi, R.; Aricò, G.; Gramowski-Voss, A.; Schroeder, O.H.; Marrazzo, A.; Prezzavento, O.; Ronsisvalle, S.; Scoto, G.M.; Ronsisvalle, G.; et al. The multitarget opioid ligand LP1’s effects in persistent pain and in primary cell neuronal cultures. Neuropharmacology 2013, 71, 70–82. [Google Scholar] [CrossRef] [PubMed]
- Parenti, C; Aricò, G; Pennisi, M; Venditti, A; Scoto, G.M. Harpagophytum procumbens extract potentiates morphine antinociception in neuropathic rats. Nat. Prod. Res. 2015. [Google Scholar] [CrossRef] [PubMed]
- Baghdikian, B.; Lanhers, M.C.; Fleurentin, J.; Ollivier, E.; Maillard, C.; Balansard, G.; Mortier, F. An analytical study, anti-inflammatory and analgesic effects of Harpagophytum procumbens and Harpagophytum zeyheri. Planta Med. 1997, 63, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Mahomed, I.M.; Ojewole, J.A. Analgesic, antiinflammatory and antidiabetic properties of Harpagophytum procumbens DC (Pedaliaceae) secondary root aqueous extract. Phytother. Res. 2004, 18, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Mahomed, I.M.; Ojewole, J.A. Anticonvulsant activity of Harpagophytum procumbens DC [Pedaliaceae] secondary root aqueous extract in mice. Brain Res. Bull. 2006, 69, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Hirai, K.; Hatanaka, J.; Hanato, J.; Umegaki, K.; Yamada, S. Antinociceptive effects of St. John’s wort, Harpagophytum procumbens extract and Grape seed proanthocyanidins extract in mice. Biol. Pharm. Bull. 2008, 31, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Bijjem, K.R.; Sharma, P.L. Anti-inflammatory and antihyperalgesic effects of the combination of ibuprofen and hemin in adjuvant-induced arthritis in the Wistar rat. Inflammopharmacology 2011, 19, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Do Val, D.R.; Bezerra, M.M.; Silva, A.A.; Pereira, K.M.; Rios, L.C.; Lemos, J.C.; Arriaga, N.C.; Vasconcelos, J.N.; Benevides, N.M.; Pinto, V.P.; et al. Tephrosiatoxicaria Pers. reduces temporomandibular joint inflammatory hypernociception: the involvement of the HO-1 pathway. Eur. J. Pain 2014, 18, 1280–1289. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, L.F.; Peroza, L.R.; Boligon, A.A.; Athayde, M.L.; Alves, S.H.; Fachinetto, R.; Wagner, C. Harpagophytum procumbens prevents oxidative stress and loss of cellviability in vitro. Neurochem. Res. 2013, 38, 2256–2267. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Park, S.K.; Zhou, J.L.; Taglialatela, G.; Chung, K.; Coggeshall, R.E.; Chung, J.M. Reactive oxygen species (ROS) play an important role in a rat model of neuropathic pain. Pain 2004, 111, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, M.; Alipieva, K.; Pashova, S.; Denev, P.; Angelova, M.; Kerns, G.; Bley, T. Antioxidant activity of devil’s claw cell biomass and its active constituents. Food Chem. 2010, 121, 967–972. [Google Scholar] [CrossRef]
- Suttner, D.M.; Sridhar, K.; Lee, C.S.; Tomura, T.; Hansen, T.N.; Dennery, P.A. Protective effects of transient HO-1 overexpression on susceptibility to oxygen toxicity in lung cells. Am. J. Physiol. 1999, 276, 443–451. [Google Scholar]
- Grosser, N.; Abate, A.; Oberle, S.; Vreman, H.J.; Dennery, P.A.; Becker, J.C.; Pohle, T.; Seidman, D.S.; Schröder, H. Heme oxygenase-1 induction may explain the antioxidant profile of aspirin. Biochem. Biophys. Res. Commun. 2003, 308, 956–960. [Google Scholar] [CrossRef]
- Yoo, S.J.; Nakra, N.K.; Ronnett, G.V.; Moon, C. Protective Effects of Inducible HO-1 on Oxygen Toxicity in Rat Brain Endothelial Microvessel Cells. Endocrinol. Metab. (Seoul) 2014, 29, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Dixon, W.J. Efficient analysis of experimental observations. Annu. Rev. Pharmacol. Toxicol. 1980, 20, 441–462. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parenti, C.; Aricò, G.; Chiechio, S.; Di Benedetto, G.; Parenti, R.; Scoto, G.M. Involvement of the Heme-Oxygenase Pathway in the Antiallodynic and Antihyperalgesic Activity of Harpagophytum procumbens in Rats. Molecules 2015, 20, 16758-16769. https://doi.org/10.3390/molecules200916758
Parenti C, Aricò G, Chiechio S, Di Benedetto G, Parenti R, Scoto GM. Involvement of the Heme-Oxygenase Pathway in the Antiallodynic and Antihyperalgesic Activity of Harpagophytum procumbens in Rats. Molecules. 2015; 20(9):16758-16769. https://doi.org/10.3390/molecules200916758
Chicago/Turabian StyleParenti, Carmela, Giuseppina Aricò, Santina Chiechio, Giulia Di Benedetto, Rosalba Parenti, and Giovanna M. Scoto. 2015. "Involvement of the Heme-Oxygenase Pathway in the Antiallodynic and Antihyperalgesic Activity of Harpagophytum procumbens in Rats" Molecules 20, no. 9: 16758-16769. https://doi.org/10.3390/molecules200916758
APA StyleParenti, C., Aricò, G., Chiechio, S., Di Benedetto, G., Parenti, R., & Scoto, G. M. (2015). Involvement of the Heme-Oxygenase Pathway in the Antiallodynic and Antihyperalgesic Activity of Harpagophytum procumbens in Rats. Molecules, 20(9), 16758-16769. https://doi.org/10.3390/molecules200916758