Benz[c,d]indolium-containing Monomethine Cyanine Dyes: Synthesis and Photophysical Properties
Abstract
:1. Introduction

2. Results and Discussion
2.1. Synthesis

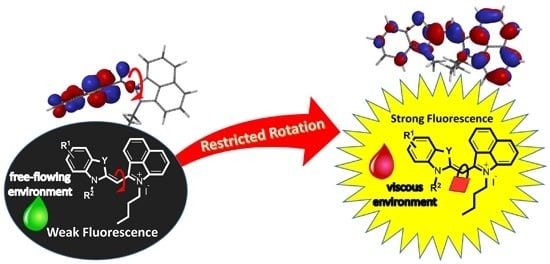
2.2. Optical Properties
| Dye | λabs (nm) a | λabs (nm) b | λemission (nm) b | Stokes Shift (nm) b | ε (M−1·cm−1) a |
|---|---|---|---|---|---|
| 6a | 498 | 505 | 570 | 65 | 37600 |
| 6b | 555 | 563 | 609 | 46 | 32300 |
| 6c | 585 | 587 | 609 | 22 | 36500 |
| 6d | 553 | 557 | 625 | 68 | 25300 |
| 10a | 537 | 552 | 657 | 105 | 33300 |
| 10b | 563 | 569 | 606 | 37 | 24800 |
| 10c | 552 | 571 | 662 | 91 | 30100 |

2.3. Computational Evaluations




| Heterocycle Included in Monomethine Dye | λabs (nm) exp. | λabs (nm) calc. | Charge of Methine Carbon | Methine Carbon Shift (ppm) | Methine Proton Shift (ppm) | N-CH3 1H Shift (ppm) | |
|---|---|---|---|---|---|---|---|
| 6a | benzoxazole | 498 | 490 | −0.535 | 75.51 | 6.15 | 4.05 |
| 6b | benzothiazole | 555 | 500 | −0.421 | 87.40 | 6.47 | 4.16 |
| 6c | quinoline | 585 | 520 | −0.526 | 93.65 | 6.35 | 4.37 |
| 6d | benz(e)indole | 553 | 487 | −0.284 | 94.10 | 6.43 | 3.60 |
| 10a | 3,3-dimethylindole | 542 | 519 | −0.328 | 82.78 | 6.30 | 3.47 |
| Substitution at the 5-position of heterocycle 10a | |||||||
| 10a | H | 542 | 519 | −0.328 | 82.78 | 6.31 | - |
| 10b | OMe | 563 | 550 | −0.316 | 83.44 | 6.23 | - |
| 10c | Cl | 552 | 530 | −0.344 | 83.81 | 6.29 | - |
2.4. DNA Binding


3. Experimental
3.1. General Information
3.2. Synthesis
3.2.1. General Synthetic Procedure for the Indolium Salts 4 and 9a–c
3.2.2. General Synthesis of the Monomethine Dyes
3.3. Stock Solutions for Optical Measurements
3.4. Method of Determining Absorbance and Fluorescence
3.5. Computational Methods
3.6. DNA Binding Studies
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mishra, A.; Behera, R.K.; Behera, P.K.; Mishra, B.K.; Behera, G.B. Cyanines during the 1990s: A Review. Chem. Rev. 2000, 100, 1973–2012. [Google Scholar] [CrossRef] [PubMed]
- Pisoni, D.S.; Todeschini, L.; Borges, A.C.A.; Petzhold, C.L.; Rodembusch, F.S.; Campo, L.F. Symmetrical and Asymmetrical Cyanine Dyes. Synthesis, Spectral Properties, and BSA Association Study. J. Org. Chem. 2014, 79, 5511–5520. [Google Scholar] [CrossRef] [PubMed]
- Wada, H.; Hyun, H.; Vargas, C.; Gravier, J.; Park, G.; Gioux, S.; Frangioni, J.V.; Henary, M.; Choi, H.S. Pancreas-Targeted NIR Fluorophores for Dual-Channel Image-Guided Abdominal Surgery. Theranostics 2015, 5, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Wada, H.; Bao, K.; Gravier, J.; Yadav, Y.; Laramie, M.; Henary, M.; Frangioni, J.V.; Choi, H.S. Phosphonated Near-Infrared Fluorophores for Biomedical Imaging of Bone. Angew. Chem. Int. Ed. Engl. 2014, 53, 10668–10672. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Owens, E.A.; Wada, H.; Levitz, A.; Park, G.; Park, M.H.; Frangioni, J.V.; Henary, M.; Choi, H.S. Cartilage-Specific Near-Infrared Fluorophores for Biomedical Imaging. Angew. Chem. Int. Ed. Engl. 2015, 54, 8648–8652. [Google Scholar] [CrossRef] [PubMed]
- Njiojob, C.N.; Owens, E.A.; Narayana, L.; Hyun, H.; Choi, H.S.; Henary, M. Tailored Near-Infrared Contrast Agents for Image Guided Surgery. J. Med. Chem. 2015, 58, 2845–2854. [Google Scholar] [CrossRef] [PubMed]
- Hyun, H.; Park, M.H.; Owens, E.A.; Wada, H.; Henary, M.; Handgraaf, H.J.M.; Vahrmeijer, A.L.; Frangioni, J.V.; Choi, H.S. Structure-inherent targeting of near-infrared fluorophores for parathyroid and thyroid gland imaging. Nat. Med. 2015, 21, 192–197. [Google Scholar] [CrossRef] [PubMed]
- El-Shishtawy, R.M.; Asiri, A.M.; Basaif, S.A.; Rashad Sobahi, T. Synthesis of a new beta-naphthothiazole monomethine cyanine dye for the detection of DNA in aqueous solution. Spectrochim. Acta A 2010, 75, 1605–1609. [Google Scholar] [CrossRef] [PubMed]
- Henary, M.; Levitz, A. Synthesis and applications of unsymmetrical carbocyanine dyes. Dyes Pigment. 2013, 99, 1107–1116. [Google Scholar] [CrossRef]
- Silva, G.L.; Ediz, V.; Yaron, D.; Armitage, B.A. Experimental and Computational Investigation of Unsymmetrical Cyanine Dyes: Understanding Torsionally Responsive Fluorogenic Dyes. J. Am. Chem. Soc. 2007, 129, 5710–5718. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wu, T.; Hu, C.; Liu, T.; Sun, W.; Fan, J.; Peng, X. The nature of the different environmental sensitivity of symmetrical and unsymmetrical cyanine dyes: An experimental and theoretical study. Phys. Chem. Chem. Phys. 2012, 14, 13702–13708. [Google Scholar] [CrossRef] [PubMed]
- Deligeorgiev, T.G.; Gadjev, N.I.; Drexhage, K.-H.; Sabnis, R.W. Preparation of intercalating dye thiazole orange and derivatives. Dyes Pigments 1995, 29, 315–322. [Google Scholar] [CrossRef]
- Thompson, M. Synthesis, Photophysical Effects, and DNA Targeting Properties of Oxazole Yellow-Peptide Bioconjugates. Bioconjugate Chem. 2006, 17, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Dähne, S.; Resch-Genger, U.; Wolfbeis, O.S. Near-Infrared Dyes for High Technology Applications; Daehne, S., Resch-Genger, U., Wolfbeis, O.S., Eds.; Kluwer: Dordrecht, The Netherland; Boston, MA, USA, 1998. [Google Scholar]
- Nygren, J.; Svanvik, N.; Kubista, M. The interactions between the fluorescent dye thiazole orange and DNA. Biopolymers 1998, 46, 39–51. [Google Scholar] [CrossRef]
- Joseph, M.J.; Taylor, J.C.; McGown, L.B.; Pitner, B.; Linn, C.P. Spectroscopic studies of YO and YOYO fluorescent dyes in a thrombin-binding DNA ligand. Biospectroscopy 1996, 2, 173–183. [Google Scholar] [CrossRef]
- Kabatc, J. Multicationic monomethine dyes as sensitizers in two- and three-component photoinitiating systems for multiacrylate monomers. J. Photochem. Photobiol. A 2010, 214, 74–85. [Google Scholar] [CrossRef]
- Hirons, G.T.; Fawcett, J.J.; Crissman, H.A. Toto and Yoyo—New Very Bright Fluorochromes for DNA Content Analyses by Flow-Cytometry. Cytometry 1994, 15, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Dietzek, B.; Brüggemann, B.; Persson, P.; Yartsev, A. On the excited-state multi-dimensionality in cyanines. Chem. Phys. Lett. 2008, 455, 13–19. [Google Scholar] [CrossRef]
- Soriano, E.; Outler, L.; Owens, E.A.; Henary, M. Synthesis of Asymmetric Monomethine Cyanine Dyes with Red-Shifted Optical Properties. J. Heterocycl. Chem. 2015, 52, 180–184. [Google Scholar] [CrossRef]
- Upadhyayula, S.; Nunez, V.; Espinoza, E.M.; Larsen, J.M.; Bao, D.D.; Shi, D.W.; Mac, J.T.; Anvari, B.; Vullev, V.I. Photoinduced dynamics of a cyanine dye: Parallel pathways of non-radiative deactivation involving multiple excited-state twisted transients. Chem. Sci. 2015, 6, 2237–2251. [Google Scholar] [CrossRef]
- Sameiro, M.; Goncalves, T. Fluorescent Labeling of Biomolecules with Organic Probes. Chem. Rev. 2009, 109, 190–212. [Google Scholar]
- Lavis, L.D.; Raines, R.T. Bright ideas for chemical biology. ACS Chem. Biol. 2008, 3, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Mann, S. Life as a nanoscale phenomenon. Angew. Chem. Int. Ed. Engl. 2008, 47, 5306–5320. [Google Scholar] [CrossRef] [PubMed]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.K.; Cheng, Z.; Behlke, M.A.; Tsourkas, A. Assessing the sensitivity of commercially available fluorophores to the intracellular environment. Anal. Chem. 2008, 80, 7437–7444. [Google Scholar] [CrossRef] [PubMed]
- Simeonov, A.; Jadhav, A.; Thomas, C.J.; Wang, Y.; Huang, R.; Southall, N.T.; Shinn, P.; Smith, J.; Austin, C.P.; Auld, D.S.; Inglese, J. Fluorescence spectroscopic profiling of compound libraries. J. Med. Chem. 2008, 51, 2363–2371. [Google Scholar] [CrossRef] [PubMed]
- Longmire, M.R.; Ogawa, M.; Hama, Y.; Kosaka, N.; Regino, C.A.; Choyke, P.L.; Kobayashi, H. Determination of optimal rhodamine fluorophore for in vivo optical imaging. Bioconjugate Chem. 2008, 19, 1735–1742. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, N.; Johnsson, K. Chemical tools for biomolecular imaging. ACS Chem. Biol. 2007, 2, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Marti, A.A.; Jockusch, S.; Stevens, N.; Ju, J.; Turro, N.J. Fluorescent hybridization probes for sensitive and selective DNA and RNA detection. Acc. Chem. Res. 2007, 40, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Willis, R.C. Portraits of life, one molecule at a time. Anal. Chem. 2007, 79, 1785–1788. [Google Scholar] [CrossRef] [PubMed]
- Hama, Y.; Urano, Y.; Koyama, Y.; Bernardo, M.; Choyke, P.L.; Kobayashi, H. A comparison of the emission efficiency of four common green fluorescence dyes after internalization into cancer cells. Bioconjugate Chem. 2006, 17, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Yuste, R. Fluorescence microscopy today. Nat. Methods 2005, 2, 902–904. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, J.W.; Fraser, S.E. The neuronal naturalist: Watching neurons in their native habitat. Nat. Neurosci. 2001, 4, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Selvin, P.R. The renaissance of fluorescence resonance energy transfer. Nat. Struct. Biol. 2000, 7, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Owens, E.A.; Su, H.; Yan, L.; Levitz, A.; Zhao, X.; Henary, M.; Zheng, Y.G. Exploration of Cyanine Compounds as Selective Inhibitors of Protein Arginine Methyltransferases: Synthesis and Biological Evaluation. J. Med. Chem. 2015, 58, 1228–1243. [Google Scholar] [CrossRef] [PubMed]
- Nanjunda, R.; Owens, E.; Mickelson, L.; Dost, T.; Stroeva, E.; Huynh, H.; Germann, M.; Henary, M.; Wilson, W. Selective G-Quadruplex DNA Recognition by a New Class of Designed Cyanines. Molecules 2013, 18, 13588–13607. [Google Scholar] [CrossRef] [PubMed]
- Mapp, C.T.; Owens, E.A.; Henary, M.; Grant, K.B. Oxidative cleavage of DNA by pentamethine carbocyanine dyes irradiated with long-wavelength visible light. Bioorganic Med. Chem. Lett. 2014, 24, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Kantor, G.J.; Hull, D.R. An effect of ultraviolet light on RNA and protein synthesis in nondividing human diploid fibroblasts. Biophys. J. 1979, 27, 359–370. [Google Scholar] [CrossRef]
- Cerutti, P.A. Prooxidant states and tumor promotion. Science 1985, 227, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Marks, R. An overview of skin cancers. Incidence and causation. Cancer 1995, 75, 607–612. [Google Scholar] [CrossRef]
- Krasnaya, Z.A.; Tret’yakova, E.O.; Kachala, V.V.; Zlotin, S.G. Synthesis of conjugated polynitriles by the reactions of β-dimethylaminoacrolein aminal and 1-dimethylamino-1,3,3-trimethoxypropane with 2-dicyanomethylene-4,5,5-trimethyl-3-cyano-2,5-dihydrofuran. Mendeleev Commun. 2007, 17, 349–351. [Google Scholar] [CrossRef]
- Brooker, L.G.S.; Keyes, G.H.; Williams, W.W. Color and Constitution. V.1 The Absorption of Unsymmetrical Cyanines. Resonance as a Basis for a Classification of Dyes. J. Am. Chem. Soc. 1942, 64, 199–210. [Google Scholar] [CrossRef]
- Williams, C.G. XXVI.—Researches on Chinoline and its Homologues. Earth Environ. Sci. Trans. R. Soc. Edinb. 1857, 21, 377–401. [Google Scholar] [CrossRef]
- Yarmoluk, S.M.; Kovalska, V.B.; Losytskyy, M.Y. Symmetric cyanine dyes for detecting nucleic acids. Biotech. Histochem. 2008, 83, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Levitz, A.; Ladani, S.T.; Hamelberg, D.; Henary, M. Synthesis and effect of heterocycle modification on the spectroscopic properties of a series of unsymmetrical trimethine cyanine dyes. Dyes Pigments 2014, 105, 238–249. [Google Scholar] [CrossRef]
- Murphy, S.; Yang, X.Q.; Schuster, G.B. Cyanine Borate Salts That Form Penetrated Ion-Pairs in Benzene Solution—Synthesis, Properties, and Structure. J. Org. Chem. 1995, 60, 2411–2422. [Google Scholar] [CrossRef]
- Deligeorgiev, T.G.; Zaneva, D.A.; Kim, S.H.; Sabnis, R.W. Preparation of monomethine cyanine dyes for nucleic acid detection. Dyes Pigments 1998, 37, 205–211. [Google Scholar] [CrossRef]
- Timcheva, I.I.; Maximova, V.A.; Deligeorgiev, T.G.; Gadjev, N.I.; Sabnis, R.W.; Ivanov, I.G. Fluorescence spectral characteristics of novel asymmetric monomethine cyanine dyes in nucleic acid solutions. FEBS Lett. 1997, 405, 141–144. [Google Scholar] [CrossRef]
- Oster, G.; Nishijima, Y. Fluorescence and Internal Rotation: Their Dependence on Viscosity of the Medium1. J. Am. Chem. Soc. 1956, 78, 1581–1584. [Google Scholar] [CrossRef]
- Potts, K.T. Heteropentalenes, in Chemistry of Heterocyclic Compounds: Special Topics in Heterocyclic Chemistry; Weissberger, A., Taylor, E.C., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1977; Volume 30. [Google Scholar]
- Escobedo, J.O.; Rusin, O.; Lim, S.; Strongin, R.M. NIR dyes for bioimaging applications. Curr. Opin. Chem. Biol. 2010, 14, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H. A Quantum-Mechanical Theory of Light Absorption of Organic Dyes and Similar Compounds. J. Chem. Phys. 1949, 17, 1198–1212. [Google Scholar] [CrossRef]
- Bickelhaupt, F.M.; Baerends, E.J. Kohn-Sham Density Functional Theory: Predicting and Understanding Chemistry. Rev. Comput. Chem. 2000, 15, 1–86. [Google Scholar]
- Cantor, C.R.; Schimmel, P.R. Biophysical Chemistry; W.H. Freeman: San Francisco, CA, USA, 1980. [Google Scholar]
- Shaikh, S.A.; Ahmed, S.R.; Jayaram, B. A molecular thermodynamic view of DNA-drug interactions: A case study of 25 minor-groove binders. Arch. Biochem. Biophys. 2004, 429, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Chaires, J.B.; Ren, J.; Hamelberg, D.; Kumar, A.; Pandya, V.; Boykin, D.W.; Wilson, W.D. Structural selectivity of aromatic diamidines. J. Med. Chem. 2004, 47, 5729–5742. [Google Scholar] [CrossRef] [PubMed]
- Fairley, T.A.; Tidwell, R.R.; Donkor, I.; Naiman, N.A.; Ohemeng, K.A.; Lombardy, R.J.; Bentley, J.A.; Cory, M. Structure, DNA minor groove binding, and base pair specificity of alkyl- and aryl-linked bis(amidinobenzimidazoles) and bis(amidinoindoles). J. Med. Chem. 1993, 36, 1746–1753. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.H.; Owens, E.A.; Feng, Y.; Yang, Y.; Xie, Y.; Tu, Y.; Henary, M.; Zheng, Y.G. Synthesis and evaluation of carbocyanine dyes as PRMT inhibitors and imaging agents. Eur. J. Med. Chem. 2012, 54, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Nanjunda, R.; Owens, E.A.; Mickelson, L.; Alyabyev, S.; Kilpatrick, N.; Wang, S.; Henary, M.; Wilson, W.D. Halogenated pentamethine cyanine dyes exhibiting high fidelity for G-quadruplex DNA. Bioorg. Med. Chem. 2012, 20, 7002–7011. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, N.; Patonay, G. A New Method for the Synthesis of Heptamethine Cyanine Dyes: Synthesis of New Near-Infrared Fluorescent Labels. J. Org. Chem. 1995, 60, 2391–2395. [Google Scholar] [CrossRef]
- Shao, Y.; Molnar, L.F.; Jung, Y.; Kussmann, J.; Ochsenfeld, C.; Brown, S.T.; Gilbert, A.T.B.; Slipchenko, L.V.; Levchenko, S.V.; O’Neill, D.P.; et al. Advances in methods and algorithms in a modern quantum chemistry program package. Phys. Chem. Chem. Phys. 2006, 8, 3172–3191. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.G.; Netz, P.A. Docking Studies on DNA-Ligand Interactions: Building and Application of a Protocol To Identify the Binding Mode. J. Chem. Inf. Model. 2009, 49, 1925–1935. [Google Scholar] [CrossRef] [PubMed]
- Kulchin, Y.N.; Vitrik, O.B.; Kamenev, O.T.; Romashko, R.V. Vector Fields Reconstruction by Fiber Optic Measuring Network; SPIE: Bellingham, WA, USA; 2001; pp. 100–108. [Google Scholar]
- Oostenbrink, C.; Soares, T.; van der Vegt, N.A.; van Gunsteren, W. Validation of the 53A6 GROMOS force field. Eur. Biophys. J. 2005, 34, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, J.; Marsili, M. Iterative partial equalization of orbital electronegativity—A rapid access to atomic charges. Tetrahedron 1980, 36, 3219–3228. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soriano, E.; Holder, C.; Levitz, A.; Henary, M. Benz[c,d]indolium-containing Monomethine Cyanine Dyes: Synthesis and Photophysical Properties. Molecules 2016, 21, 23. https://doi.org/10.3390/molecules21010023
Soriano E, Holder C, Levitz A, Henary M. Benz[c,d]indolium-containing Monomethine Cyanine Dyes: Synthesis and Photophysical Properties. Molecules. 2016; 21(1):23. https://doi.org/10.3390/molecules21010023
Chicago/Turabian StyleSoriano, Eduardo, Cory Holder, Andrew Levitz, and Maged Henary. 2016. "Benz[c,d]indolium-containing Monomethine Cyanine Dyes: Synthesis and Photophysical Properties" Molecules 21, no. 1: 23. https://doi.org/10.3390/molecules21010023
APA StyleSoriano, E., Holder, C., Levitz, A., & Henary, M. (2016). Benz[c,d]indolium-containing Monomethine Cyanine Dyes: Synthesis and Photophysical Properties. Molecules, 21(1), 23. https://doi.org/10.3390/molecules21010023