Antibacterial Activity and Antibiotic-Enhancing Effects of Honeybee Venom against Methicillin-Resistant Staphylococcus aureus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of BV on MRSA
| MRSA Strains | MIC (µg/mL) | MBC (µg/mL) |
|---|---|---|
| CCARM 3366 | 0.085 ± 0.003 | 0.106 ± 0.006 |
| CCARM 3708 | 0.11 ± 0.001 | 0.14 ± 0.009 |
2.2. Time-Kill Studies

2.3. Synergistic Effects of BV on MRSA
| Strains | Agent | MIC | FIC (µg/mL) | FIC Index a | Outcome | |
|---|---|---|---|---|---|---|
| Alone | Combination | |||||
| CCARM 3366 | Ampicillin BV | 435 | 1.06 | 0.002 | 1.002 | indifferent |
| 0.085 | 0.085 | 1.00 | ||||
| Penicillin BV | 680 | 1.33 | 0.001 | 0.631 | partial synergy | |
| 0.085 | 0.054 | 0.63 | ||||
| Gentamicin BV | 0.5 | 0.025 | 0.05 | 0.14 | synergy | |
| 0.085 | 0.008 | 0.09 | ||||
| Vancomycin BV | 2 | 0.55 | 0.27 | 0.48 | synergy | |
| 0.085 | 0.018 | 0.21 | ||||
| CCARM 3708 | Ampicillin BV | 540 | 1.33 | 0.002 | 1.002 | indifferent |
| 0.11 | 0.11 | 1.00 | ||||
| Penicillin BV | 851 | 1.75 | 0.002 | 0.772 | partial synergy | |
| 0.11 | 0.085 | 0.77 | ||||
| Gentamicin BV | 0.44 | 0.028 | 0.063 | 0.373 | synergy | |
| 0.11 | 0.035 | 0.31 | ||||
| Vancomycin BV | 1.7 | 0.70 | 0.41 | 0.72 | partial synergy | |
| 0.11 | 0.035 | 0.31 | ||||
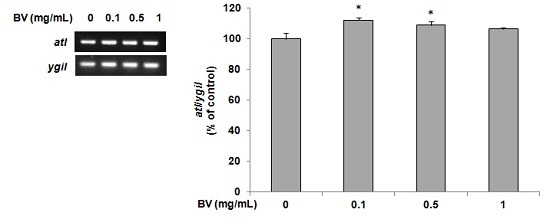
2.4. Determination of atl Gene Expression

2.5. Discussion
3. Experimental Section
3.1. General Information
3.1.1. Bee Venom
3.1.2. Bacterial Strains
3.2. Evaluation of Anti-Bacterial Activity
3.2.1. Minimum Inhibitory Concentrations (MIC)
3.2.2. Minimum Bactericidal Concentrations (MBC)
3.2.3. Time-Kill Assays
3.3. Synergistic Effects of BV on MRSA
3.4. RNA Isolation and RT-PCR
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Iwamoto, M.; Mu, Y.; Lynfield, R.; Bulens, S.N.; Nadle, J.; Aragon, D.; Petit, S.; Ray, S.M.; Harrison, L.H.; Dumyati, G.; et al. Trends in invasive methicillin-resistant Staphylococcus aureus infections. Pediatrics 2013, 132, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Kang, M.D.; Hwang, E.J.; Eom, S.H.; Yang, J.Y.; Lee, M.S.; Lee, W.J.; Jeon, Y.J.; Choi, J.S.; Kim, Y.M. Synergistic effect between dieckol from Ecklonia stolonifera and β-lactams against methicillin-resistant Staphylococcus aureus. Biotechnol. Bioprocess Eng. 2008, 13, 758–764. [Google Scholar] [CrossRef]
- Hiramatsu, K.; Hanaki, H.; Ino, T.; Yabuta, K.; Oguri, T.; Tenover, F.C. Methicillin-resistant Staphylocccus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 1997, 40, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.L.; Lehman, M.K.; Fey, P.D.; Bayles, K.W. Contribution of the Staphylococcus aureus Atl AM and GL murein hydrolase activities in cell division, autolysis, and biofilm formation. PLoS ONE 2012, 7, e42244. [Google Scholar] [CrossRef] [PubMed]
- Somerfield, S.D.; Stach, J.L.; Mraz, C.; Gervais, F.; Skamene, E. Bee venom inhibits superoxide production by human neutrophils. Inflammation 1984, 8, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Castro, H.J.; Mendez-Lnocencio, J.I.; Omidvar, B.; Omidvar, J.; Santilli, J.; Nielsen, H.S.; Pavot, A.P.; Richert, J.R.; Bellanti, J.A. A phase I study of the safety of honeybee venom extract as a possible treatment for patients with progressive forms of multiple sclerosis. Allergy Asthma Proc. 2005, 26, 470–476. [Google Scholar] [PubMed]
- Son, D.J.; Lee, J.W.; Lee, Y.H.; Song, H.S.; Lee, C.K.; Hong, J.T. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol. Ther. 2007, 115, 246–270. [Google Scholar] [CrossRef] [PubMed]
- Lariviere, W.R.; Melzack, R. The bee venom test: A new tonic-pain test. Pain 1996, 66, 271–277. [Google Scholar] [CrossRef]
- Han, S.M.; Lee, K.G.; Yeo, J.H.; Kweon, H.Y.; Woo, S.O.; Lee, M.Y.; Baek, H.J.; Park, K.K. Inhibitory effect of bee venom against ultraviolet B induced MMP-1 and MMP-3 in human dermal fibroblasts. J. Apic. Res. 2007, 46, 94–98. [Google Scholar] [CrossRef]
- Han, S.M.; Lee, K.G.; Yeo, J.H.; Baek, H.J.; Park, K.K. Antibacterial and anti-inflammatory effects of honeybee (Apis mellifera) venom against acne1-inducing bacteria. J. Med. Plant Res. 2010, 4, 459–464. [Google Scholar]
- Keary, R.; Gaitero, M.S.; van Raaij, M.J.; O’Mahony, J.; Fenton, M.; McAuliffe, O.; Colin, H.; Paul Ross, R.; Coffey, A. Characterization of a bacteriophage-derived murein peptidase for elimination of antibiotic-resistant Staphylococcus aureus. Curr. Protein Pept. Sci. 2015, 16. [Google Scholar] [CrossRef]
- Kang, S.Y.; Roh, D.H.; Yoon, S.Y.; Moon, J.Y.; Kim, H.W.; Lee, H.J.; Beitz, A.J.; Lee, J.H. Repetitive treatment with diluted bee venom reduces neuropathic pain via potentiation of locus coeruleus noradrenergic neuronal activity and modulation of spinal NR1 phosphorylation in rats. J. Pain 2012, 13, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Ip, S.W.; Liao, S.S.; Lin, S.Y.; Lin, J.P.; Yang, J.S.; Lin, M.L.; Han, S.M.; Chung, J.G. The role of mitochondria in bee venom-induced apoptosis in human breast cancer MCF7 cells. In Vivo 2008, 22, 237–245. [Google Scholar] [PubMed]
- Park, M.H.; Choi, M.S.; Kwak, D.H.; Oh, K.W.; Yoon, D.Y.; Han, S.B.; Song, H.S.; Song, M.J.; Hong, J.T. Anti-cancer effect of bee venom in prostate cancer cells through activation of caspase pathway via inactivation of NF-κB. Prostate 2011, 71, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Lee, K.G.; Yeo, J.H.; Kim, W.T.; Park, K.K. Biological effects of treatment of an animal skin wound with honeybee (Apis mellifera. L) venom. J. Plast. Reconstr. Aesthet. Surg. 2011, 64, e67–e72. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.G.; Cho, H.J.; Bae, Y.S.; Park, K.K.; Choe, J.Y.; Chung, I.K.; Kim, M.; Yeo, J.H.; Park, K.H.; Lee, Y.S.; et al. Bee venom suppresses LPS-mediated NO/iNOS induction through inhibition of PKC-alpha expression. J. Ethnopharmacol. 2009, 123, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Lee, K.G.; Yeo, J.H.; Kweon, H.Y.; Woo, S.O.; Lee, M.Y.; Baek, H.J.; Park, K.K. Effect of venom from the Asian honeybee (Apis cerana Fab.) on LPS-induced nitric oxide and tumor necrosis factor-α production in RAW 264.7 cell line. J. Apic. Res. 2006, 45, 131–136. [Google Scholar] [CrossRef]
- Climo, M.W.; Patron, R.L.; Archer, G.L. Combinations of vancomycin and beta-lactams are synergistic against staphylococci with reduced susceptibilities to vancomycin. Antimicrob. Agents Chemother. 1999, 43, 1747–1753. [Google Scholar] [PubMed]
- Eliopoulos, G.M.; Moellering, R.C. Antimicrobial combinations. In Antibiotics in Laboratory Medicine, 5th ed.; Lorian, V., Ed.; Williams & Wilkins Co: Baltimore, MD, USA, 2005; pp. 432–492. [Google Scholar]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Sugai, M.; Komatsuzawa, H.; Nakashima, S.; Oshida, T.; Matsumoto, A.; Suginaka, H. An autolysin ring associated with cell separation of Staphylococcus aureus. J. Bacteriol. 1996, 178, 1568–1571. [Google Scholar]
- Mates, S.M.; Patel, L.; Kaback, H.R.; Miller, M.H. Membrane potential in anaerobically growing Staphylococcus aureus and its relationship to gentamicin uptake. Antimicrob. Agents Chemother. 1983, 23, 526–530. [Google Scholar] [CrossRef] [PubMed]
- National Committee for Clinical Laboratory Standards (NCCLS). Performance Standards for Antimicrobial Disk Susceptibility Testing; 14th Informational Supplement. NCCLS Document M100-S14; NCCLS: Wayne, PA, USA, 2004. [Google Scholar]
- National Committee for Clinical Laboratory Standards (NCCLS). Performance Standards for Antimicrobial Susceptibility Testing; 18th Informational Supplement. NCCLS Document M100-S18; NCCLS: Wayne, PA, USA, 2008. [Google Scholar]
- Shahverdi, A.R.; Fakhimi, A.; Zarrini, G.; Dehghan, G.; Iranshahi, M. Galbanic acid from Ferula szowitsiana enhanced the antibacterial activity of penicillin G and cephalexin against Staphylococcus aureus. Biol. Pharm. Bull. 2007, 30, 1805–1807. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.; Burton, N.; Cooper, R. Manuka honey inhibits cell division in methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2011, 66, 2536–2542. [Google Scholar]
- Sample Availability: Samples of honeybee venom are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.M.; Kim, J.M.; Hong, I.P.; Woo, S.O.; Kim, S.G.; Jang, H.R.; Pak, S.C. Antibacterial Activity and Antibiotic-Enhancing Effects of Honeybee Venom against Methicillin-Resistant Staphylococcus aureus. Molecules 2016, 21, 79. https://doi.org/10.3390/molecules21010079
Han SM, Kim JM, Hong IP, Woo SO, Kim SG, Jang HR, Pak SC. Antibacterial Activity and Antibiotic-Enhancing Effects of Honeybee Venom against Methicillin-Resistant Staphylococcus aureus. Molecules. 2016; 21(1):79. https://doi.org/10.3390/molecules21010079
Chicago/Turabian StyleHan, Sang Mi, Joung Min Kim, In Pyo Hong, Soon Ok Woo, Se Gun Kim, He Rye Jang, and Sok Cheon Pak. 2016. "Antibacterial Activity and Antibiotic-Enhancing Effects of Honeybee Venom against Methicillin-Resistant Staphylococcus aureus" Molecules 21, no. 1: 79. https://doi.org/10.3390/molecules21010079
APA StyleHan, S. M., Kim, J. M., Hong, I. P., Woo, S. O., Kim, S. G., Jang, H. R., & Pak, S. C. (2016). Antibacterial Activity and Antibiotic-Enhancing Effects of Honeybee Venom against Methicillin-Resistant Staphylococcus aureus. Molecules, 21(1), 79. https://doi.org/10.3390/molecules21010079