Terminamines K–S, Antimetastatic Pregnane Alkaloids from the Whole Herb of Pachysandra terminalis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Purification and Characterization
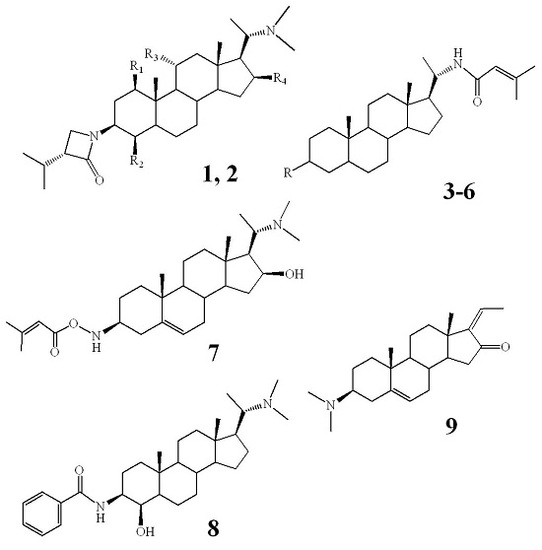
2.2. Structural Elucidation of Compounds 1–9
3. Materials and Methods
3.1. General
3.2. Plant Materials
3.3. Extraction and Isolation
3.4. Chemotaxis Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rahman, A.; Choudhary, M.I. Chapter 2 Chemistry and Biology of Steroidal Alkaloids. In The Alkaloids: Chemistry and Biology; Cordell, G.A., Ed.; Academic Press: London, UK, 1998; Volume 50, pp. 61–108. [Google Scholar]
- Ata, A.; Andersh, B.J. Chapter 3 Buxus Steroidal Alkaloids: Chemistry and Biology. In The Alkaloids: Chemistry and Biology; Cordell, G.A., Ed.; Academic Press: London, UK, 2008; Volume 66, pp. 191–213. [Google Scholar]
- Funayama, S.; Noshita, T.; Shinoda, K.; Haga, N.; Nozoe, S.; Hayashi, M.; Komiyama, K. Cytotoxic alkaloids of Pachysandra terminalis. Biol. Pharm. Bull. 2000, 23, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Watanabe, K.; Watanabe, H.; Shimizu, M.; Kikuchi, T. Effects on gastric acid secretion of a steroidal alkaloid, epipachysamine-A, extracted from Pachysandra terminalis Sieb. et Zucc. J. Pharm. Dyn. 1984, 7, 263–267. [Google Scholar] [CrossRef]
- Osori, E.J.; Robledo, S.M.; Bastida, J. Chapter 2 Alkaloids with Antiprotozoal Activity. In The Alkaloids: Chemistry and Biology; Cordell, G.A., Ed.; Academic Press: London, UK, 2008; Volume 66, pp. 113–190. [Google Scholar]
- Sun, Y.; Yan, Y.X.; Chen, J.C.; Lu, L.; Zhang, X.M.; Li, Y.; Qiu, M.H. Pregnane alkaloids from Pachysandra axillaries. Steroids 2010, 75, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Zhai, H.Y.; Zhao, C.; Zhang, N.; Jin, M.N.; Tang, S.A.; Qin, N.; Kong, D.X.; Duan, H.Q. Alkaloids from Pachysandra terminalis inhibit breast cancer invasion and have potential for the development of anti-metastasis therapeutic agents. J. Nat. Prod. 2012, 75, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Gan, C.C.; Jin, M.N.; Tang, S.A.; Qin, N.; Duan, H.Q. Antitumor metastasis pregnane alkaloids form Pachysandra terminalis. J. Asian Nat. Prod. Res. 2014, 16, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.Z.; Wang, F.; Yang, L.J.; Zhang, G.L. Pregnane alkaloids from Sarcococca hookeriana var. digyna. Fitoterapia 2013, 89, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Uyeo, S. Pachysandra alkaloids. III. Structures of pachysandrin B, -C, and -D. Chem. Pharm. Bull. 1967, 15, 207–213. [Google Scholar] [CrossRef]
- Kikuchi, T.; Uyeo, S.; Nishinaga, T. Studies on the alkaloids of Pachysandra terminals Sieb. et Zucc. (8): Structure of epipachysandrine-A. Tetrahedron Lett. 1966, 7, 1749–1752. [Google Scholar] [CrossRef]
- Sanchez, V.; Ahond, A.; Guihem, J.; Poupat, C.; Potier, P. Alcaloides des feuilles de Didymeles madagascariensis Willd., des feuilles et des ecorces de racines de Didymeles perrieri Leandri (Didymelacees). Bull. Soc. Chim. Fr. 1987, 5, 877–884. [Google Scholar]
- Atta-ur-Rahman; Choudhary, M.I.; Khan, M.R.; Iqbal, M.Z. Three new steroidal amines from Sarcococca saligna. Nat. Prod. Lett. 1998, 11, 81–91. [Google Scholar] [CrossRef]
- Chiu, M.H.; Nie, R.L.; Li, Z.R.; Zhou, J. Isospiropachysine, a steroidal alkaloid from Pachysandra axillaries. Phytochemistry 1990, 29, 3927–3930. [Google Scholar]
- Yan, Y.X.; Sun, Y.; Chen, J.C.; Wang, Y.Y.; Li, Y.; Qiu, M.H. Cytotoxic steroids from Sarcococca saligna. Planta Med. 2011, 77, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.E.; Roach, S.L. A convenient two-step procedure for the synthesis of substituted allylic amines from allylic alcohols. Synthesis 1995, 7, 756–758. [Google Scholar] [CrossRef]
- Liu, J.; Ma, S.N.; Zhang, X.; Jin, M.N.; Jin, M.H.; Kong, D.X.; Qin, N.; Duan, H.Q. Synthesis and antimetastatic effect of E-salignone. Chem. Nat. Compd. 2014, 50, 697–701. [Google Scholar] [CrossRef]
- Qin, N.; Jia, M.; Wu, X.R.; Shou, X.A.; Liu, Q.; Gan, C.C.; Jin, M.N.; Yu, Y.; Duan, H.Q. Synthesis and anti-metastatic effects of pregn-17(20)-2n-3-amine-derivatives. Eur. J. Med. Chem. 2016, 124, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Qin, N.; Liu, J.; Jin, M.N.; Zhang, X.; Jin, M.H.; Kong, D.X.; Jiang, S.D.; Duan, H.Q. Synthesis and antimetastatic effects of E-salignone amide derivatives. Drug Dev. Res. 2014, 75, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Qiu, M.H.; Wang, D.Z.; Nie, R.L. Study on 13C-NMR spectroscopy of Pachysandra alkaloids. Chin. J. Magn. Reson. 1995, 12, 155–165. [Google Scholar]
- Wan, W.Z.; Zou, H.X.; Sun, R.H.; Liu, Y.; Wang, J.N.; Ma, D.L.; Zhang, N. Investigate the role of PTEN in chemotaxis of human breast cancer cells. Cell Signal. 2007, 19, 2227–2236. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.H.; Gao, P.; Chen, L.; Ma, D.L.; Wang, J.M.; Oppenheim, J.J.; Zhang, N. Protein kinase C zeta is required for epidermal growth factor-induced chemotaxis of human breast cancer cells. Cancer Res. 2005, 65, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Vlahos, C.J.; Matter, W.F.; Hui, K.Y.; Brown, R.F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). Biol. Chem. 1994, 7, 5241–5248. [Google Scholar]
- Sample Availability: Not available.




| Position | Terminamine K (1) | Terminamine L (2) | ||
|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 74.6 | 4.95, dd (11.2, 4.8) | 36.5 | 1.96, m |
| 1.54, m | ||||
| 2 | 28.1 | 1.70, m | 27.6 | 1.93, m |
| 2.33, m | 1.55, m | |||
| 3 | 48.0 | 4.18, m | 58.5 | 4.42, m |
| 4 | 72.2 | 4.55, dd (12.0, 4.0) | 207.6 | - |
| 5 | 44.2 | 1.70, m | 58.2 | 2.16, m |
| 6 | 22.9 | 1.33, m | 20.3 | 1.62, m |
| 1.82, m | 1.44, m | |||
| 7 | 32.1 | 1.00, m | 30.2 | 1.75, m |
| 1.88, m | 0.81, m | |||
| 8 | 34.1 | 1.69, m | 34.9 | 1.30, m |
| 9 | 56.6 | 1.41, m | 54.2 | 0.96, m |
| 10 | 42.2 | - | 42.5 | - |
| 11 | 71.4 | 5.05, m | 21.6 | 1.55, m |
| 1.26, m | ||||
| 12 | 45.0 | 2.60, m | 39.6 | 1.91, m |
| 0.80, m | 1.20, m | |||
| 13 | 41.2 | 41.7 | ||
| 14 | 51.4 | 0.99, m | 54.7 | 1.40, m |
| 15 | 35.1 | 2.21, m | 24.0 | 1.62, m |
| 1.19, m | 1.08, m | |||
| 16 | 72.5 | 4.33, m | 27.5 | 1.92, m |
| 17 | 58.8 | 1.21, m | 56.1 | 1.09, m |
| 18 | 14.4 | 0.86, s | 12.3 | 0.65, s |
| 19 | 10.6 | 1.15, s | 13.8 | 0.75, s |
| 20 | 56.6 | 2.89, m | 61.3 | 2.54, m |
| 21 | 9.8 | 0.89, d (6.4) | 10.0 | 0.90, d (6.4) |
| N(Me)2 | 39.8 | 2.23, s | 39.9 | 2.21, s |
| 2′ | 170.1 | - | 165.1 | - |
| 3′ | 56.2 | 3.06, m | 130.9 | - |
| 4′ | 45.4 | 3.70, m | 45.8 | 3.93, d (6.5) |
| 3.18, m | 3.68, d (6.5) | |||
| 5′ | 27.5 | 2.06, m | 135.1 | - |
| 5′-(Me)2 | 19.8 | 1.03, d (6.7) | 19.9 | 2.04, s |
| 19.7 | 1.07, d (6.7) | 20.3 | 1.70, s | |
| 1-OAc | 21.9 | 1.94, s | ||
| 170.8 | ||||
| 4-OAc | 21.0 | 2.05, s | ||
| 170.8 | ||||
| 11-Val | 176.3 | |||
| 26.5 | 1.43, m | |||
| 1.65, m | ||||
| 40.2 | 2.31, m | |||
| 14.1 | 1.04, d (7.6) | |||
| 10.8 | 0.89, d (7.6) | |||
| Position | Terminamine M (3) | Terminamine N (4) | Terminamine O (5) | Terminamine P (6) | ||||
|---|---|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 37.6 | 1.79, m | 38.3 | 1.90, m | 37.6 | 1.90, m | 33.3 | 1.30, m |
| 0.96, m | 1.06, m | 1.06, m | 1.60, m | |||||
| 2 | 24.0 | 1.57, m | 24.0 | 1.60, m | 24.0 | 1.60, m | 25.0 | 1.74, m |
| 1.09, m | 1.13, m | 1.11, m | ||||||
| 3 | 64.4 | 2.47, m | 64.9 | 2.23, m | 59.5 | 2.59, m | 54.9 | 2.81, m |
| 4 | 32.0 | 0.87, m | 35.2 | 2.24, m | 37.3 | 2.35, m | 36.4 | 2.48, m |
| 1.67, m | 2.12, m | |||||||
| 5 | 45.6 | 1.09, m | 142.0 | - | 140.0 | - | 139.0 | - |
| 6 | 30.5 | 1.55, m | 120.7 | 5.35, m | 121.9 | 5.37, m | 122.9 | 5.35, m |
| 1.32, m | ||||||||
| 7 | 28.9 | 1.28, m | 31.9 | 1.99, m | 31.8 | 1.99, m | 31.8 | 1.95, m |
| 1.53, m | 1.50, m | 1.58, m | ||||||
| 8 | 35.3 | 1.37, m | 31.7 | 1.49, m | 31.7 | 1.47, m | 31.7 | 1.47, m |
| 9 | 54.3 | 0.64, m | 50.2 | 0.95, m | 50.1 | 0.95, m | 50.0 | 1.09, m |
| 10 | 35.7 | - | 36.9 | - | 37.0 | - | 37.2 | - |
| 11 | 21.0 | 1.51, m | 20.8 | 1.49, m | 20.8 | 1.48, m | 20.6 | 1.48, m |
| 1.27, m | ||||||||
| 12 | 39.4 | 1.91, m | 39.2 | 1.96, m | 39.1 | 1.95, m | 39.1 | 1.94, m |
| 1.11, m | 1.16, m | 1.17, m | 1.16, m | |||||
| 13 | 42.2 | - | 41.9 | - | 41.9 | - | 41.9 | - |
| 14 | 56.6 | 1.02, m | 56.8 | 1.07, m | 56.8 | 1.03, m | 56.7 | 1.05, m |
| 15 | 24.1 | 1.82, m | 25.0 | 1.81, m | 24.0 | 1.59, m | 24.0 | 1.59, m |
| 1.47, m | 1.50, m | 1.11, m | 1.10, m | |||||
| 16 | 26.8 | 1.75, m | 26.8 | 1.78, m | 26.7 | 1.77, m | 26.8 | 1.77, m |
| 1.46, m | 1.49, m | 1.49, m | 1.47, m | |||||
| 17 | 56.9 | 1.28, m | 56.9 | 1.31, m | 56.8 | 1.31, m | 56.7 | 1.31, m |
| 18 | 12.3 | 0.72, s | 12.2 | 0.75, s | 12.2 | 0.75, s | 12.1 | 0.75, s |
| 19 | 12.4 | 0.77, s | 19.4 | 0.98, s | 19.3 | 1.00, s | 19.1 | 1.02, s |
| 20 | 47.2 | 4.05, m | 47.2 | 4.06, m | 47.2 | 4.05, m | 47.2 | 4.05, m |
| 21 | 21.9 | 1.16, d (6.3) | 21.9 | 1.17, d (6.8) | 21.9 | 1.17, d (6.4) | 21.9 | 1.17, d (6.5) |
| N(Me)2 | 41.3 | 2.36, s | 41.6 | 2.35, s | 31.7 | 2.49, s | 33.1 | 2.38, s |
| NH | - | 5.12, d (9.1) | - | 5.13, d (8.8) | - | 5.13, d (8.8) | - | 5.21, d (8.1) |
| 2′ | 165.8 | - | 165.8 | - | 165.8 | - | 165.8 | - |
| 3′ | 119.0 | 5.49, s | 119.0 | 5.49, s | 119.0 | 5.49, s | 119.1 | 5.50, s |
| 4′ | 150.0 | - | 150.0 | - | 150.0 | - | 149.9 | - |
| 4′-(Me)2 | 19.7 | 2.13, s | 19.7 | 2.14, s | 19.7 | 2.14, s | 19.7 | 2.14, s |
| 27.1 | 1.82, s | 27.1 | 1.82, s | 27.1 | 1.82, s | 27.1 | 1.82, s | |
| Position | Terminamine Q (7) | Terminamine R (8) | Terminamine S (9) | |||
|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 37.9 | 1.84, m | 32.8 | 1.60, m | 35.6 | 1.88, m |
| 1.17, m | 1.10, m | 1.38, m | ||||
| 2 | 29.3 | 1.87, m | 27.1 | 2.15, m | 20.6 | 1.72, m |
| 1.35, m | 1.89, m | 1.62, m | ||||
| 3 | 49.3 | 3.73, m | 51.2 | 4.47, m | 65.1 | 2.15, m |
| 4 | 39.4 | 2.34, m | 71.1 | 3.78, m | 32.0 | 1.63, m |
| 2.09, m | ||||||
| 5 | 140.3 | - | 47.4 | 1.22, m | 142.3 | - |
| 6 | 121.7 | 5.38, m | 29.7 | 1.26, m | 120.2 | 5.34, br d (4.8) |
| 7 | 31.9 | 2.02, m | 31.2 | 1.78, m | 31.7 | 1.99, m |
| 1.54, m | 0.89, m | |||||
| 8 | 31.3 | 1.57, m | 34.5 | 1.41, m | 30.9 | 1.65, m |
| 9 | 50.1 | 0.98, m | 54.4 | 0.77, m | 49.8 | 1.40, m |
| 10 | 36.6 | - | 37.2 | - | 37.1 | - |
| 11 | 20.7 | 1.47, m | 20.7 | 1.54, m | 20.5 | 1.72, m |
| 1.29, m | 1.61, m | |||||
| 12 | 40.0 | 1.84, m | 39.7 | 1.90, m | 35.2 | 2.23, m |
| 1.13, m | 1.20, m | |||||
| 13 | 41.4 | - | 42.5 | - | 43.0 | - |
| 14 | 53.6 | 0.92, m | 56.1 | 1.08, m | 50.8 | 1.46, m |
| 15 | 34.9 | 2.18, m | 24.3 | 1.76, m | 39.5 | 2.20, m |
| 1.25, m | 1.25, m | 39.5 | 2.03, m | |||
| 16 | 72.6 | 4.36, m | 24.9 | 1.92, m | 208.7 | - |
| 17 | 58.9 | 1.26, m | 52.5 | 1.47, m | 148.2 | - |
| 18 | 14.1 | 0.89, s | 12.3 | 0.69, s | 19.4 | 0.94, s |
| 19 | 19.4 | 1.00, s | 13.0 | 0.88, s | 19.4 | 1.03, s |
| 20 | 56.8 | 2.97, m | 64.0 | 3.21, m | 130.2 | 5.73, q (7.2) |
| 21 | 9.9 | 0.95, d (6.4) | 12.7 | 1.32, d (6.0) | 14.0 | 2.09, d (7.2) |
| N(Me)2 | 39.9 | 2.25, s | 39.4 | 2.48, s | 41.7 | 2.31, s |
| NH | - | 5.20, d (8.0) | - | 6.38, d (6.7) | ||
| 2′ | 166.2 | - | 170 | - | ||
| 3′ | 118.8 | 5.52, s | 134.4 | - | ||
| 4′ | 150.4 | - | 127.1 | 7.80, d (7.2) | ||
| 5′ | 128.6 | 7.46, m | ||||
| 6′ | 131.7 | 7.53, m | ||||
| 7′ | 128.6 | 7.46, m | ||||
| 8′ | 127.1 | 7.80, d (7.2) | ||||
| 4′-(Me)2 | 19.7 | 2.15, s | ||||
| 27.1 | 1.83, s | |||||
| Compound | IC50 a (μM) | Compound | IC50 a (μM) |
|---|---|---|---|
| 1 | 0.58 ± 0.06 | 10 | 2.35 ± 0.31 |
| 2 | 4.65 ± 0.82 | 11 | 5.71 ± 0.84 |
| 3 | 3.14 ± 0.51 | 12 | 1.91 ± 0.15 |
| 4 | 8.12 ± 0.75 | 13 | 3.32 ± 0.28 |
| 5 | 0.71 ± 0.06 | 14 | 2.16 ± 0.23 |
| 6 | 6.47 ± 0.54 | 15 | 3.98 ± 0.36 |
| 7 | 1.38 ± 0.17 | 16 | 6.91 ± 0.74 |
| 8 | 8.62 ± 1.03 | 17 | 0.31 ± 0.01 |
| 9 | 1.01 ± 0.09 | ||
| LY294002 b | 1.18 ± 0.14 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.-Y.; Yu, Y.; Jia, M.; Jin, M.-N.; Qin, N.; Zhao, C.; Duan, H.-Q. Terminamines K–S, Antimetastatic Pregnane Alkaloids from the Whole Herb of Pachysandra terminalis. Molecules 2016, 21, 1283. https://doi.org/10.3390/molecules21101283
Li X-Y, Yu Y, Jia M, Jin M-N, Qin N, Zhao C, Duan H-Q. Terminamines K–S, Antimetastatic Pregnane Alkaloids from the Whole Herb of Pachysandra terminalis. Molecules. 2016; 21(10):1283. https://doi.org/10.3390/molecules21101283
Chicago/Turabian StyleLi, Xiang-Yu, Yang Yu, Miao Jia, Mei-Na Jin, Nan Qin, Chuan Zhao, and Hong-Quan Duan. 2016. "Terminamines K–S, Antimetastatic Pregnane Alkaloids from the Whole Herb of Pachysandra terminalis" Molecules 21, no. 10: 1283. https://doi.org/10.3390/molecules21101283
APA StyleLi, X. -Y., Yu, Y., Jia, M., Jin, M. -N., Qin, N., Zhao, C., & Duan, H. -Q. (2016). Terminamines K–S, Antimetastatic Pregnane Alkaloids from the Whole Herb of Pachysandra terminalis. Molecules, 21(10), 1283. https://doi.org/10.3390/molecules21101283