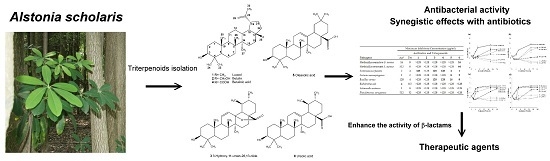
Antibacterial and Synergistic Activity of Pentacyclic Triterpenoids Isolated from Alstonia scholaris
Abstract
:1. Introduction
2. Results
2.1. Isolation and Identification of Triterpenoids from A. scholaris

| Pathogens | Fractions | |||||
|---|---|---|---|---|---|---|
| Hex * | EA | BuOH | Aq | EA-8 | EA-12 | |
| Methicillin-sensitive S. aureus | 0 # | 10 | 8 | 7 | 8 | 12 |
| Enterococcus faecalis | 0 | 10 | 8 | 8 | 10 | 12 |
| Listeria monocytogenes | 0 | 12 | 10 | 7 | 8 | 10 |
| Bacillus cereus | 0 | 16 | 12 | 7 | 14 | 22 |
| Escherichia coli | 0 | 0 | 0 | 0 | 0 | 0 |
| Salmonella enterica | 0 | 0 | 0 | 0 | 0 | 0 |
| Pseudomonas aeruginosa | 0 | 0 | 0 | 0 | 0 | 0 |
2.2. The Minimal Inhibitory Concentrations (MICs) of Triterpenoids on Bacterial Pathogens
| Pathogens | Minimum Inhibitory Concentration (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| Antibiotics and Triterpenoids | ||||||||
| Ap * | Tet | 1 | 2 | 3 | 4 | 5 | 6 | |
| Methicillin-sensitive S. aureus | 16 | 8 | >128 | >128 | >128 | >128 | >128 | 16 |
| Methicillin-resistant S. aureus | 512 | 8 | >128 | >128 | >128 | >128 | >128 | 64 |
| Enterococcus faecalis | 2 | 4 | 128 | >128 | 128 | 128 | 4 | 1 |
| Listeria monocytogenes | 1 | 2 | >128 | >128 | >128 | >128 | 8 | 2 |
| Bacillus cereus | 128 | 4 | >128 | >128 | 128 | 128 | 16 | 8 |
| Escherichia coli | 4 | 0.5 | >128 | >128 | >128 | >128 | >128 | >128 |
| Salmonella enterica | 1 | 8 | >128 | >128 | >128 | >128 | >128 | >128 |
| Pseudomonas aeruginosa | 512 | 32 | >128 | >128 | >128 | >128 | >128 | >128 |
2.3. Evaluation of Synergistic Effects
| Pathogens | Agents | FICA | FICB | FICI | Outcome |
|---|---|---|---|---|---|
| MSSA | UA + Amp | 0.25 | 0.125 | 0.375 | Synergy |
| UA + Tet | 0.125 | 0.0625 | 0.188 | Synergy | |
| MRSA | UA + Amp | 0.25 | 0.125 | 0.375 | Synergy |
| UA + Tet | 0.0625 | 0.031 | 0.093 | Synergy | |
| B. cereus | UA + Amp | 0.25 | 0.031 | 0.281 | Synergy |
| UA + Tet | 0.125 | 0.125 | 0.25 | Synergy | |
| OA + Amp | 0.125 | 0.0625 | 0.188 | Synergy | |
| OA + Tet | 0.015 | 0.062 | 0.078 | Synergy | |
| E. faecalis | UA + Amp | 0.5 | 0.25 | 0.725 | Indifferent |
| UA + Tet | 0.125 | 0.5 | 0.625 | Indifferent | |
| OA + Amp | 1 | 1 | 2 | Indifferent | |
| OA + Tet | 1 | 1 | 2 | Indifferent | |
| L. monocytogenes | UA + Amp | 0.5 | 0.0625 | 0.563 | Indifferent |
| UA + Tet | 0.0625 | 0.0625 | 0.125 | Synergy | |
| OA + Amp | 1 | 1 | 2 | Indifferent | |
| OA + Tet | 1 | 0.5 | 1.5 | Indifferent |
2.4. Time-Kill Curve Assay


3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Bioassay Guided Chromatography
4.3. Isolation and Identification of Triterpenoids
4.4. Bacterial Strains and Media
4.5. Minimal Inhibitory Concentration (MIC) Determination
4.6. Synergistic Effects of Drug Combination
4.7. Time-Kill Assay
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baliga, M.S. Review of the phytochemical, pharmacological and toxicological properties of Alstonia scholaris Linn. R. Br (Saptaparna). Chin. J. Integr. Med. 2012, 18. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.H.; Cai, X.H.; Zhao, Y.L.; Feng, T.; Luo, X.D. Pharmacological evaluation of Alstonia scholaris: Anti-tussive, anti-asthmatic and expectorant activities. J. Ethnopharmacol. 2010, 129, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Khyade, M.S.; Kasote, D.M.; Vaikos, N.P. Alstonia scholaris (L.) R. Br. and Alstonia macrophylla Wall. ex G. Don: A comparative review on traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2014, 153. [Google Scholar] [CrossRef] [PubMed]
- El-Askary, H.I.; El-Olemy, M.M.; Salama, M.M.; Sleem, A.A.; Amer, M.H. Bioguided isolation of pentacyclic triterpenes from the leaves of Alstonia scholaris (Linn.) R. Br. growing in Egypt. Nat. Prod. Res. 2012, 26, 1755–1758. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ren, F.C.; Liu, J.K. Alstonic acids A and B, unusual 2,3-secofernane triterpenoids from Alstonia scholaris. Phytochemistry 2009, 70, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.H.; Tan, Q.G.; Liu, Y.P.; Feng, T.; Du, Z.Z.; Li, W.Q.; Luo, X.D. A cage-monoterpene indole alkaloid from Alstonia scholaris. Org. Lett. 2008, 10, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.; Vinayak, V.K. Preliminary evaluation of extracts of Alstonia scholaris bark for in vivo antimalarial activity in mice. J. Ethnopharmacol. 1990, 29, 51–57. [Google Scholar] [CrossRef]
- Lin, S.C.; Lin, C.C.; Lin, Y.H.; Supriyatna, S.; Pan, S.L. The protective effect of Alstonia scholaris R. Br. on hepatotoxin-induced acute liver damage. Am. J. Chin. Med. 1996, 24, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Baliga, M.S. Evaluation of anticancer activity of the alkaloid fraction of Alstonia scholaris (Sapthaparna) in vitro and in vivo. Phytother. Res. 2006, 20, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.H.; Cai, X.H.; Feng, T.; Zhao, Y.L.; Wang, J.K.; Zhang, L.Y.; Yan, M.; Luo, X.D. Pharmacological evaluation of Alstonia scholaris: Anti-inflammatory and analgesic effects. J. Ethnopharmacol. 2010, 129, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Saraswathi, V.; Ramamoorthy, N.; Subramaniam, S.; Mathuram, V.; Gunasekaran, P.; Govindasamy, S. Inhibition of glycolysis and respiration of sarcoma-180 cells by echitamine chloride. Chemotherapy 1998, 44, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Bonvicini, F.; Mandrone, M.; Antognoni, F.; Poli, F.; Gentilomi, G.A. Ethanolic extracts of Tinospora cordifolia and Alstonia scholaris show antimicrobial activity towards clinical isolates of methicillin-resistant and carbapenemase-producing bacteria. Nat. Prod. Res. 2014, 28, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Lamb, A.J. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents 2011, 38, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Kurek, A.; Nadkowska, P.; Pliszka, S.; Wolska, K.I. Modulation of antibiotic resistance in bacterial pathogens by oleanolic acid and ursolic acid. Phytomedicine 2012, 19, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Walencka, E.; Rozalska, S.; Wysokinska, H.; Rozalski, M.; Kuzma, L.; Rozalska, B. Salvipisone and aethiopinone from Salvia sclarea hairy roots modulate staphylococcal antibiotic resistance and express anti-biofilm activity. Planta Med. 2007, 73, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Brehm-Stecher, B.F.; Johnson, E.A. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol, and apritone. Antimicrob. Agents Chemother. 2003, 47, 3357–3360. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52. [Google Scholar] [CrossRef] [PubMed]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Dieases (ESCMID). Teriminology relating to methods for determination of susceptibility of bacteria to antimicrobial agents. Clin. Microbiol. Infec. 2000, 6, 503–508. [Google Scholar]
- Moellering, R.C., Jr. MRSA: The first half century. J. Antimicrob. Chemother. 2012, 67, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Stefani, S.; Chung, D.R.; Lindsay, J.A.; Friedrich, A.W.; Kearns, A.M.; Westh, H.; Mackenzie, F.M. Meticillin-resistant Staphylococcus aureus (MRSA): Global epidemiology and harmonisation of typing methods. Int. J. Antimicrob. Agents 2012, 39, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Bottone, E.J. Bacillus cereus, a volatile human pathogen. Clin. Microbiol. Rev. 2010, 23, 382–398. [Google Scholar] [CrossRef] [PubMed]
- Kiyomizu, K.; Yagi, T.; Yoshida, H.; Minami, R.; Tanimura, A.; Karasuno, T.; Hiraoka, A. Fulminant septicemia of Bacillus cereus resistant to carbapenem in a patient with biphenotypic acute leukemia. J. Infect. Chemother. 2008, 14, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Kudaka, J.; Horikawa, K.; Uryu, K.; Matsuyuki, S.; Ogata, K.; Kawano, K.; Yamaguchi, Y.; Yamasaki, S.; Watanabe, H.; Iwanaga, M. Symptoms of food-borne diseases and gastroenteritis in Kyushu, Japan. Kansenshogaku Zasshi 2005, 79, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.M.; Chen, T.H. Bacterial foodborne outbreaks in central Taiwan, 1991–2000. J. Food Drug Anal. 2003, 11, 53–59. [Google Scholar]
- Cho, J.I.; Lee, S.H.; Lim, J.S.; Koh, Y.J.; Kwak, H.S.; Hwang, I.G. Detection and distribution of food-borne bacteria in ready-to-eat foods in Korea. Food Sci. Biotechnol. 2011, 20, 525–529. [Google Scholar] [CrossRef]
- Hall, J.A.; Goulding, J.S.; Bean, N.H.; Tauxe, R.V.; Hedberg, C.W. Epidemiologic profiling: Evaluating foodborne outbreaks for which no pathogen was isolated by routine laboratory testing: United States, 1982–1989. Epidemiol. Infect. 2001, 127, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [PubMed]
- Tsutsumi, L.S.; Owusu, Y.B.; Hurdle, J.G.; Sun, D.Q. Progress in the discovery of treatments for C. difficile Infection: A clinical and medicinal chemistry review. Curr. Top. Med. Chem. 2014, 14, 152–175. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Chang, F.R.; Wang, H.K.; Park, Y.K.; Ikegaki, M.; Kilgore, N.; Lee, K.H. Anti-AIDS agents. 48.(1) Anti-HIV activity of moronic acid derivatives and the new melliferone-related triterpenoid isolated from Brazilian propolis. J. Nat. Prod. 2001, 64, 1278–1281. [Google Scholar] [CrossRef] [PubMed]
- Kurek, A.; Grudniak, A.M.; Szwed, M.; Klicka, A.; Samluk, L.; Wolska, K.I.; Janiszowska, W.; Popowska, M. Oleanolic acid and ursolic acid affect peptidoglycan metabolism in Listeria monocytogenes. Antonie van Leeuwenhoek 2010, 97, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Chen, H.T.; Li, T.C.; Weng, J.H.; Jhan, Y.L.; Lin, S.X.; Chou, C.H. The role of pentacyclic triterpenoids in the allelopathic effects of Alstonia scholaris. J. Chem. Ecol. 2014, 40, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Way, T.D.; Tsai, S.J.; Wang, C.M.; Ho, C.T.; Chou, C.H. Chemical constituents of Rhododendron formosanum show pronounced growth inhibitory effect on non-small-cell lung carcinoma cells. J. Agric. Food Chem. 2014, 62, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Frere, J.M. Mechanism of action of beta-lactam antibiotics at the molecular level. Biochem. Pharmacol. 1977, 26, 2203–2210. [Google Scholar] [CrossRef]
- De Leon, L.; Beltran, B.; Moujir, L. Antimicrobial activity of 6-oxophenolic triterpenoids. Mode of action against Bacillus subtilis. Planta Med. 2005, 71, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Nagasaki, S.; Ohta, T. Sesquiterpene farnesol inhibits recycling of the C55 lipid carrier of the murein monomer precursor contributing to increased susceptibility to beta-lactams in methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2007, 59, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Jhan, Y.L.; Yen, L.S.; Su, Y.H.; Chang, C.C.; Wu, Y.Y.; Chang, C.I.; Tsai, S.Y.; Chou, C.H. The allelochemicals of litchi leaf and its potential as natural herbicide in weed control. Allelopath. J. 2013, 32, 157–174. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 10th ed.; CLSI document M07-A10; CLSI: Wayne, PA, USA, 2015. [Google Scholar]
- Wang, C.M.; Hsu, Y.M.; Jhan, Y.L.; Tsai, S.J.; Lin, S.X.; Su, C.H.; Chou, C.H. Structure elucidation of procyanidins isolated from Rhododendron formosanum and their anti-oxidative and anti-bacterial activities. Molecules 2015, 20, 12787–12803. [Google Scholar] [CrossRef] [PubMed]
- Hemaiswarya, S.; Doble, M. Synergistic interaction of eugenol with antibiotics against Gram negative bacteria. Phytomedicine 2009, 16, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–6 are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.-M.; Chen, H.-T.; Wu, Z.-Y.; Jhan, Y.-L.; Shyu, C.-L.; Chou, C.-H. Antibacterial and Synergistic Activity of Pentacyclic Triterpenoids Isolated from Alstonia scholaris. Molecules 2016, 21, 139. https://doi.org/10.3390/molecules21020139
Wang C-M, Chen H-T, Wu Z-Y, Jhan Y-L, Shyu C-L, Chou C-H. Antibacterial and Synergistic Activity of Pentacyclic Triterpenoids Isolated from Alstonia scholaris. Molecules. 2016; 21(2):139. https://doi.org/10.3390/molecules21020139
Chicago/Turabian StyleWang, Chao-Min, Hsiao-Ting Chen, Zong-Yen Wu, Yun-Lian Jhan, Ching-Lin Shyu, and Chang-Hung Chou. 2016. "Antibacterial and Synergistic Activity of Pentacyclic Triterpenoids Isolated from Alstonia scholaris" Molecules 21, no. 2: 139. https://doi.org/10.3390/molecules21020139
APA StyleWang, C. -M., Chen, H. -T., Wu, Z. -Y., Jhan, Y. -L., Shyu, C. -L., & Chou, C. -H. (2016). Antibacterial and Synergistic Activity of Pentacyclic Triterpenoids Isolated from Alstonia scholaris. Molecules, 21(2), 139. https://doi.org/10.3390/molecules21020139