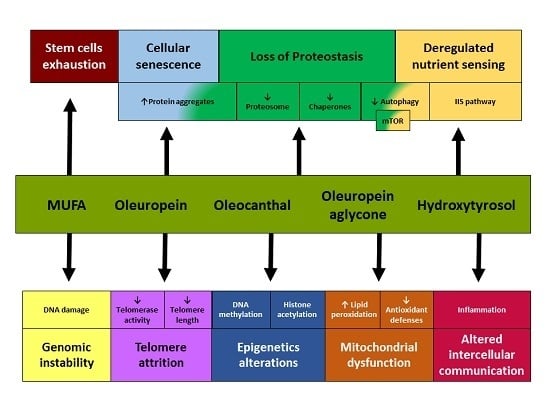
Olive Oil and the Hallmarks of Aging
Abstract
:1. Introduction
2. Olive Oil and Genomic Instability
3. Olive Oil Effect on Telomere Attrition
4. Epigenetic Changes Induced by Olive Oil
5. Olive Oil Effects on Proteostasis
6. Olive Oil and Nutrient Sensing Pathways
7. Mitochondrial Dysfunction
8. Olive Oil and Cellular Senescence
9. Effects of Olive Oil on Stem Cell Exhaustion
10. Alterations of Intercellular Communication Pathways by Olive Oil
11. Concluding Remarks and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Li, M.; Izpisua Belmonte, J.C. Ageing: Genetic rejuvenation of old muscle. Nature 2014, 506, 304–305. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Victor, P.; Gutarra, S.; Garcia-Prat, L.; Rodriguez-Ubreva, J.; Ortet, L.; Ruiz-Bonilla, V.; Jardi, M.; Ballestar, E.; Gonzalez, S.; Serrano, A.L.; et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 2014, 506, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, A.; Hamuro, J.; Nakamura, H.; Kondo, N.; Hirabayashi, Y.; Ishizaki-Koizumi, S.; Hirakawa, T.; Inoue, T.; Yodoi, J. Overexpression of human thioredoxin in transgenic mice controls oxidative stress and life span. Antioxid. Redox Signal. 2002, 4, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Schriner, S.E.; Linford, N.J.; Martin, G.M.; Treuting, P.; Ogburn, C.E.; Emond, M.; Coskun, P.E.; Ladiges, W.; Wolf, N.; Van Remmen, H.; et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 2005, 308, 1909–1911. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Weindruch, R. Oxidative stress, caloric restriction, and aging. Science 1996, 273, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Miranda, J.; Perez-Jimenez, F.; Ros, E.; De Caterina, R.; Badimon, L.; Covas, M.I.; Escrich, E.; Ordovas, J.M.; Soriguer, F.; Abia, R.; et al. Olive oil and health: Summary of the ii international conference on olive oil and health consensus report, jaen and cordoba (Spain) 2008. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Galli, C. Biological properties of olive oil phytochemicals. Crit. Rev. Food Sci. Nutr. 2002, 42, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Giovanelli, L. Beneficial effects of olive oil phenols on the aging process. Experimental evidence and possible mechanisms of action. Nutr. Aging 2012, 1, 207–223. [Google Scholar]
- Van Remmen, H.; Richardson, A. Oxidative damage to mitochondria and aging. Exp. Gerontol. 2001, 36, 957–968. [Google Scholar] [CrossRef]
- Richter, C.; Park, J.W.; Ames, B.N. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc. Natl. Acad. Sci. USA 1988, 85, 6465–6467. [Google Scholar] [CrossRef] [PubMed]
- Meissner, C.; Bruse, P.; Oehmichen, M. Tissue-specific deletion patterns of the mitochondrial genome with advancing age. Exp. Gerontol. 2006, 41, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Zapico, S.C.; Ubelaker, D.H. Relationship between mitochondrial DNA mutations and aging. Estimation of age-at-death. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Moraes, C.T. Double-strand breaks of mouse muscle mtdna promote large deletions similar to multiple mtdna deletions in humans. Hum. Mol. Genet. 2005, 14, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.L.; Ochoa, J.J.; Ramirez-Tortosa, C.; Battino, M.; Huertas, J.R.; Martin, Y.; Mataix, J. Dietary fat type (virgin olive vs. Sunflower oils) affects age-related changes in DNA double-strand-breaks, antioxidant capacity and blood lipids in rats. Exp. Gerontol. 2004, 39, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.L.; Ochoa, J.J.; Ramirez-Tortosa, C.; Huertas, J.R.; Mataix, J. Age-related mtdna deletion in rat liver depends on dietary fat unsaturation. J. Gerontol. 2006, 61A, 107–114. [Google Scholar] [CrossRef]
- Fabiani, R.; Rosignoli, P.; de Bartolomeo, A.; Fuccelli, R.; Servili, M.; Montedoro, G.F.; Morozzi, G. Oxidative DNA damage is prevented by extracts of olive oil, hydroxytyrosol, and other olive phenolic compounds in human blood mononuclear cells and HL60 cells. J. Nutr. 2008, 138, 1411–1416. [Google Scholar] [PubMed]
- Miro-Casas, E.; Covas, M.I.; Fito, M.; Farre-Albadalejo, M.; Marrugat, J.; de la Torre, R. Tyrosol and hydroxytyrosol are absorbed from moderate and sustained doses of virgin olive oil in humans. Eur. J. Clin. Nutr. 2003, 57, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Erol, O.; Arda, N.; Erdem, G. Phenols of virgin olive oil protects nuclear DNA against oxidative damage in hela cells. Food Chem. Toxicol. 2012, 50, 3475–3479. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H. Structure and function of telomeres. Nature 1991, 350, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Blasco, M.A. Telomere length, stem cells and aging. Nat. Chem. Biol. 2007, 3, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Blasco, M.A. Telomeres and human disease: Ageing, cancer and beyond. Nat. Rev. Genet. 2005, 6, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Calado, R.T.; Young, N.S. Telomere diseases. N. Engl. J. Med. 2009, 361, 2353–2365. [Google Scholar] [CrossRef] [PubMed]
- Haycock, P.C.; Heydon, E.E.; Kaptoge, S.; Butterworth, A.S.; Thompson, A.; Willeit, P. Leucocyte telomere length and risk of cardiovascular disease: Systematic review and meta-analysis. BMJ 2014, 349, g4227. [Google Scholar] [CrossRef] [PubMed]
- Aviv, A.; Chen, W.; Gardner, J.P.; Kimura, M.; Brimacombe, M.; Cao, X.; Srinivasan, S.R.; Berenson, G.S. Leukocyte telomere dynamics: Longitudinal findings among young adults in the bogalusa heart study. Am. J. Epidemiol. 2009, 169, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H.; Greider, C.W.; Szostak, J.W. Telomeres and telomerase: The path from maize, tetrahymena and yeast to human cancer and aging. Nat. Med. 2006, 12, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Palm, W.; de Lange, T. How shelterin protects mammalian telomeres. Ann. Rev. Genet. 2008, 42, 301–334. [Google Scholar] [CrossRef] [PubMed]
- Diez Roux, A.V.; Ranjit, N.; Jenny, N.S.; Shea, S.; Cushman, M.; Fitzpatrick, A.; Seeman, T. Race/ethnicity and telomere length in the multi-ethnic study of atherosclerosis. Aging Cell 2009, 8, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Adler, N.; Pantell, M.S.; O’Donovan, A.; Blackburn, E.; Cawthon, R.; Koster, A.; Opresko, P.; Newman, A.; Harris, T.B.; Epel, E. Educational attainment and late life telomere length in the health, aging and body composition study. Brain Behav. Immun. 2013, 27, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.E.; Diez-Roux, A.V.; Adler, N.E.; Seeman, T.E. Socioeconomic factors and leukocyte telomere length in a multi-ethnic sample: Findings from the multi-ethnic study of atherosclerosis (MESA). Brain Behav. Immun. 2013, 28, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Kiecolt-Glaser, J.K.; Epel, E.S.; Belury, M.A.; Andridge, R.; Lin, J.; Glaser, R.; Malarkey, W.B.; Hwang, B.S.; Blackburn, E. Omega-3 fatty acids, oxidative stress, and leukocyte telomere length: A randomized controlled trial. Brain behav. Immun. 2013, 28, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Prescott, J.; Kraft, P.; Han, J.; Giovannucci, E.; Hankinson, S.E.; de Vivo, I. Physical activity, sedentary behavior, and leukocyte telomere length in women. Am. J. Epidemiol. 2012, 175, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Okereke, O.I.; Prescott, J.; Wong, J.Y.; Han, J.; Rexrode, K.M.; de Vivo, I. High phobic anxiety is related to lower leukocyte telomere length in women. PLoS ONE 2012, 7, e40516. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.; Du, M.; Wong, J.Y.Y.; Han, J.; de Vivo, I. Paternal age at birth is associated with offspring leukocyte telomere length in the nurses’ health study. Hum. Reprod. 2012, 27, 3622–3631. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, V.; Esposito, A.; Rizzo, M.R.; Marfella, R.; Barbieri, M.; Paolisso, G. Mediterranean diet, telomere maintenance and health status among elderly. PLoS ONE 2013, 8, e62781. [Google Scholar]
- Crous-Bou, M.; Fung, T.T.; Prescott, J.; Julin, B.; Du, M.; Sun, Q.; Rexrode, K.M.; Hu, F.B.; de Vivo, I. Mediterranean diet and telomere length in nurses’ health study: Population based cohort study. BMJ 2014, 349, g6674. [Google Scholar] [CrossRef] [PubMed]
- De Vos-Houben, J.M.J.; Ottenheim, N.R.; Kafatos, A.; Buijsse, B.; Hageman, G.J.; Kromhout, D.; Giltay, E.J. Telomere length, oxidative stress, and antioxidant status in elderly men in zutphen and crete. Mech. Ageing Dev. 2012, 133, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Honig, L.S.; Schupf, N.; Lee, J.H.; Luchsinger, J.A.; Stern, Y.; Scarmeas, N. Mediterranean diet and leukocyte telomere length in a multi-ethnic elderly population. Age 2015, 37, 24. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Delgado-Lista, J.; Ramirez, R.; Carracedo, J.; Caballero, J.; Perez-Martinez, P.; Gutierrez-Mariscal, F.M.; Garcia-Rios, A.; Delgado-Casado, N.; Cruz-Teno, C.; et al. Mediterranean diet reduces senescence-associated stress in endothelial cells. Age 2012, 34, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- García-Calzón, S.; Gea, A.; Razquin, C.; Corella, D.; Lamuela-Raventós, R.M.; Martínez, J.A.; Martínez-González, M.A.; Zalba, G.; Marti, A. Longitudinal association of telomere length and obesity indices in an intervention study with a mediterranean diet: The predimed-navarra trial. Int. J. Obes. 2014, 38, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Armanios, M.; Blackburn, E.H. The telomere syndromes. Nat. Rev. Genet. 2012, 13, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Parzonko, A.; Czerwinska, M.E.; Kiss, A.K.; Naruszewicz, M. Oleuropein and oleacein may restore biological functions of endothelial progenitor cells impaired by angiotensin II via activation of Nrf2/heme oxygenase-1 pathway. Phytomedicine 2013, 20, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Bird, A. Perceptions of epigenetics. Nature 2007, 447, 396–398. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Aslibekyan, S.; Irvin, M.R.; Claas, S.A.; Borecki, I.B.; Ordovas, J.M.; Absher, D.M.; Arnett, D.K. SNPs located at CpG sites modulate genome-epigenome interaction. Epigenetics 2013, 8, 802–806. [Google Scholar] [CrossRef] [PubMed]
- Campión, J.; Milagro, F.; Martinez, J.A. Epigenetics and obesity. Prog. Mol. Biol. Trans. Sci. 2009, 94, 291–347. [Google Scholar]
- Di Francesco, A.; Falconi, A.; Di Germanio, C.; di Bonaventura, M.V.M.; Costa, A.; Caramuta, S.; del Carlo, M.; Compagnone, D.; Dainese, E.; Cifani, C.; et al. Extravirgin olive oil up-regulates CB 1 tumor suppressor gene in human colon cancer cells and in rat colon via epigenetic mechanisms. J. Nutr. Biochem. 2015, 26, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Shumaker, D.K.; Dechat, T.; Kohlmaier, A.; Adam, S.A.; Bozovsky, M.R.; Erdos, M.R.; Eriksson, M.; Goldman, A.E.; Khuon, S.; Collins, F.S.; et al. Mutant nuclear lamin a leads to progressive alterations of epigenetic control in premature aging. Proc. Natl. Acad. Sci. USA 2006, 103, 8703–8708. [Google Scholar] [CrossRef] [PubMed]
- Osorio, F.G.; Varela, I.; Lara, E.; Puente, X.S.; Espada, J.; Santoro, R.; Freije, J.M.; Fraga, M.F.; Lopez-Otin, C. Nuclear envelope alterations generate an aging-like epigenetic pattern in mice deficient in Zmpste24 metalloprotease. Aging Cell 2010, 9, 947–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maegawa, S.; Hinkal, G.; Kim, H.S.; Shen, L.; Zhang, L.; Zhang, J.; Zhang, N.; Liang, S.; Donehower, L.A.; Issa, J.P. Widespread and tissue specific age-related DNA methylation changes in mice. Genome Res. 2010, 20, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Voisin, S.; Almén, M.S.; Moschonis, G.; Chrousos, G.P.; Manios, Y.; Schiöth, H.B. Dietary fat quality impacts genome-wide DNA methylation patterns in a cross-sectional study of greek preadolescents. Eur. J. Hum. Genet. 2015, 23, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean diet pyramid: A cultural model for healthy eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [PubMed]
- Martín-Núñez, G.M.; Cabrera-Mulero, R.; Rubio-Martín, E.; Rojo-Martínez, G.; Olveira, G.; Valdés, S.; Soriguer, F.; Castaño, L.; Morcillo, S. Methylation levels of the SCD1 gene promoter and LINE-1 repeat region are associated with weight change: An intervention study. Mol. Nutr. Food Res. 2014, 58, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Oliveras-Ferraros, C.; Fernández-Arroyo, S.; Vazquez-Martin, A.; Lozano-Sánchez, J.; Cufí, S.; Joven, J.; Micol, V.; Fernández-Gutiérrez, A.; Segura-Carretero, A.; Menendez, J.A. Crude phenolic extracts from extra virgin olive oil circumvent de novo breast cancer resistance to HER1/HER2-targeting drugs by inducing GADD45-sensed cellular stress, G2/M arrest and hyperacetylation of histone H3. Int. J. Oncol. 2011, 38, 1533–1547. [Google Scholar] [PubMed]
- Hoile, S.P.; Clarke-Harris, R.; Huang, R.C.; Calder, P.C.; Mori, T.A.; Beilin, L.J.; Lillycrop, K.A.; Burdge, G.C. Supplementation with N-3 long-chain polyunsaturated fatty acids or olive oil in men and women with renal disease induces differential changes in the DNA methylation of FADS2 and ELOVL5 in peripheral blood mononuclear cells. PLoS ONE 2014, 9, e109896. [Google Scholar] [CrossRef] [PubMed]
- Hodson, L.; Fielding, B.A. Stearoyl-coa desaturase: Rogue or innocent bystander? Prog. Lipid Res. 2013, 52, 15–42. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, R.W.; Jonas, W.; Ernst, S.B.; Kammel, A.; Jähnert, M.; Schürmann, A. Diet-dependent alterations of hepatic scd1 expression are accompanied by differences in promoter methylation. Horm. Metab. Res. 2013, 45, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Ariyama, H.; Kono, N.; Matsuda, S.; Inoue, T.; Arai, H. Decrease in membrane phospholipid unsaturation induces unfolded protein response. J. Biol. Chem. 2010, 285, 22027–22035. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.L.; Huertas, J.R.; Manas, M.; Battino, M.; Mataix, J. Physical exercise affects the lipid profile of mitochondrial membranes in rats fed with virgin olive oil or sunflower oil. Br. J. Nutr. 1999, 81, 21–24. [Google Scholar] [PubMed]
- Milagro, F.I.; Gómez-Abellán, P.; Campión, J.; Martínez, J.A.; Ordovás, J.M.; Garaulet, M. Clock, PER2 and BMAL1 DNA methylation: Association with obesity and metabolic syndrome characteristics and monounsaturated fat intake. Chronobiol. Int. 2012, 29, 1180–1194. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, D.P.; Cano, P.; Jiménez-Ortega, V.; Esquifino, A.I. Melatonin and the metabolic syndrome: Physiopathologic and therapeutical implications. Neuroendocrinology 2011, 93, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Santos, C.; Gómez-Abellán, P.; Madrid, J.A.; Hernández-Morante, J.J.; Lujan, J.A.; Ordovas, J.M.; Garaulet, M. Circadian rhythm of clock genes in human adipose explants. Obesity 2009, 17, 1481–1485. [Google Scholar] [CrossRef] [PubMed]
- Mazeh, H.; Mizrahi, I.; Ilyayev, N.; Halle, D.; Brücher, B.L.D.M.; Bilchik, A.; Protic, M.; Daumer, M.; Stojadinovic, A.; Avital, I.; et al. The diagnostic and prognostic role of microrna in colorectal cancer-a comprehensive review. J. Cancer 2013, 4, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. Micrornas: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Casas-Agustench, P.; Fernandes, F.S.; Tavares do Carmo, M.G.; Visioli, F.; Herrera, E.; Dávalos, A. Consumption of distinct dietary lipids during early pregnancy differentially modulates the expression of micrornas in mothers and offspring. PLoS ONE 2015, 10, e0117858. [Google Scholar]
- Corella, D.; Ordovás, J.M. How does the mediterranean diet promote cardiovascular health? Current progress toward molecular mechanisms: Gene-diet interactions at the genomic, transcriptomic, and epigenomic levels provide novel insights into new mechanisms. Bioessays 2014, 36, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular chaperones in protein folding and proteostasis. Nature 2011, 475, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Koga, H.; Kaushik, S.; Cuervo, A.M. Protein homeostasis and aging: The importance of exquisite quality control. Ageing Res. Rev. 2011, 10, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.P.; Bukau, B. Hsp70 chaperones: Cellular functions and molecular mechanism. Cell. Mol. Life Sci. 2005, 62, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Brocchieri, L.; de Macario, E.C.; Macario, A.J.L. Hsp70 genes in the human genome: Conservation and differentiation patterns predict a wide array of overlapping and specialized functions. BMC Evol. Biol. 2008, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Daugaard, M.; Rohde, M.; Jäättelä, M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007, 581, 3702–3710. [Google Scholar] [CrossRef] [PubMed]
- Neznanov, N.; Komarov, A.P.; Neznanova, L.; Stanhope-Baker, P.; Gudkov, A.V. Proteotoxic stress targeted therapy (PSTT): Induction of protein misfolding enhances the antitumor effect of the proteasome inhibitor bortezomib. Oncotarget 2011, 2, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, S.K.; Murshid, A.; Prince, T. The shock of aging: Molecular chaperones and the heat shock response in longevity and aging—A mini-review. Gerontology 2009, 55, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Morrow, G.; Samson, M.; Michaud, S.; Tanguay, R.M. Overexpression of the small mitochondrial Hsp22 extends Drosophila life span and increases resistance to oxidative stress. FASEB J. 2004, 18, 598–599. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.A.; Lithgow, G.J. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell 2003, 2, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Min, J.N.; Whaley, R.A.; Sharpless, N.E.; Lockyer, P.; Portbury, A.L.; Patterson, C. Chip deficiency decreases longevity, with accelerated aging phenotypes accompanied by altered protein quality control. Mol. Cell. Biol. 2008, 28, 4018–4025. [Google Scholar] [CrossRef] [PubMed]
- Swindell, W.R.; Masternak, M.M.; Kopchick, J.J.; Conover, C.A.; Bartke, A.; Miller, R.A. Endocrine regulation of heat shock protein mRNA levels in long-lived dwarf mice. Mech. Ageing Dev. 2009, 130, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Powers, E.T.; Morimoto, R.I.; Dillin, A.; Kelly, J.W.; Balch, W.E. Biological and chemical approaches to diseases of proteostasis deficiency. Ann. Rev. Biochem. 2009, 78, 959–991. [Google Scholar] [CrossRef] [PubMed]
- Daccache, A.; Lion, C.; Sibille, N.; Gerard, M.; Slomianny, C.; Lippens, G.; Cotelle, P. Oleuropein and derivatives from olives as tau aggregation inhibitors. Neurochem. Int. 2011, 58, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, S.; Guidotti, V.; Bucciantini, M.; Parri, M.; Nediani, C.; Cerbai, E.; Stefani, M.; Berti, A. Oleuropein aglycon prevents cytotoxic amyloid aggregation of human amylin. J. Nutr. Biochem. 2010, 21, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.K.; Rajendran, M.Y.; Monfries, C.; Hall, C.; Lim, L. The human heat-shock protein family. Expression of a novel heat-inducible Hsp70 (Hsp70b’) and isolation of its cdna and genomic DNA. Biochem. J. 1990, 267, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Leung, T.K.C.; Hall, C.; Rajendran, M.; Spurr, N.K.; Lim, L. The human heat-shock genes Hspa6 and Hspa7 are both expressed and localize to chromosome 1. Genomics 1992, 12, 74–79. [Google Scholar] [CrossRef]
- Noonan, E.J.; Place, R.F.; Giardina, C.; Hightower, L.E. Hsp70b’ regulation and function. Cell Stress Chaperones 2007, 12, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Milner, C.M.; Campbell, R.D. Structure and expression of the three MHC-linked HSP70 genes. Immunogenetics 1990, 32, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Sargent, C.A.; Dunham, I.; Trowsdale, J.; Campbell, R.D. Human major histocompatibility complex contains genes for the major heat shock protein HSP70. Proc. Natl. Acad. Sci. USA 1989, 86, 1968–1972. [Google Scholar] [CrossRef] [PubMed]
- Son, W.Y.; Hwang, S.H.; Han, C.T.; Lee, J.H.; Kim, S.; Kim, Y.C. Specific expression of heat shock protein HspA2 in human male germ cells. Mol. Hum. Reprod. 1999, 5, 1122–1126. [Google Scholar] [CrossRef] [PubMed]
- Cañuelo, A.; Gilbert-López, B.; Pacheco-Liñán, P.; Martínez-Lara, E.; Siles, E.; Miranda-Vizuete, A. Tyrosol, a main phenol present in extra virgin olive oil, increases lifespan and stress resistance in caenorhabditis elegans. Mech. Ageing Dev. 2012, 133, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Chiang, W.C.; Ching, T.T.; Lee, H.C.; Mousigian, C.; Hsu, A.L. HSF-1 regulators DDL-1/2 link insulin-like signaling to heat-shock responses and modulation of longevity. Cell 2012, 148, 322–334. [Google Scholar] [CrossRef] [PubMed]
- Hsu, A.L.; Murphy, C.T.; Kenyon, C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science 2003, 300, 1142–1145. [Google Scholar] [CrossRef] [PubMed]
- Westerheide, S.D.; Anckar, J.; Stevens, S.M., Jr.; Sistonen, L.; Morimoto, R.I. Stress-inducible regulation of heat shock factor 1 by the deacetylase SIRT1. Science 2009, 323, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.W.; Francis, R.E.; Petkovic, M. Foxo transcription factors: Key regulators of cell fate. Biochem. Soc. Trans. 2006, 34, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Arden, K.C.; Biggs, W.H., III. Regulation of the foxo family of transcription factors by phosphatidylinositol-3 kinase-activated signaling. Arch. Biochem. Biophys. 2002, 403, 292–298. [Google Scholar] [CrossRef]
- Lim, J.H.; Gerhart-Hines, Z.; Dominy, J.E.; Lee, Y.; Kim, S.; Tabata, M.; Xiang, Y.K.; Puigserver, P. Oleic acid stimulates complete oxidation of fatty acids through protein kinase A-dependent activation of sirt1-pgc1α complex. J. Biol. Chem. 2013, 288, 7117–7126. [Google Scholar] [CrossRef] [PubMed]
- Katsiki, M.; Chondrogianni, N.; Chinou, I.; Rivett, A.J.; Gonos, E.S. The olive constituent oleuropein exhibits proteasome stimulatory properties in vitro and confers life span extension of human embryonic fibroblasts. Rejuvenation Res. 2007, 10, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Bullon, P.; Battino, M.; Varela-Lopez, A.; Perez-Lopez, P.; Granados-Principal, S.; Ramirez-Tortosa, M.C.; Ochoa, J.J.; Cordero, M.D.; Gonzalez-Alonso, A.; Ramirez-Tortosa, C.L.; et al. Diets based on virgin olive oil or fish oil but not on sunflower oil prevent age-related alveolar bone resorption by mitochondrial-related mechanisms. PLoS ONE 2013, 8, e74234. [Google Scholar]
- Oliván, S.; Martínez-Beamonte, R.; Calvo, A.C.; Surra, J.C.; Manzano, R.; Arnal, C.; Osta, R.; Osada, J. Extra virgin olive oil intake delays the development of amyotrophic lateral sclerosis associated with reduced reticulum stress and autophagy in muscle of SOD1G93A mice. J. Nutr. Biochem. 2014, 25, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. Aging: Ros or tor. Cell Cycle 2008, 7, 3344–3354. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.E.; Strong, R.; Sharp, Z.D.; Nelson, J.F.; Astle, C.M.; Flurkey, K.; Nadon, N.L.; Wilkinson, J.E.; Frenkel, K.; Carter, C.S.; et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009, 460, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.E.; Burmeister, L.; Brooks, S.V.; Chan, C.C.; Friedline, S.; Harrison, D.E.; Hejtmancik, J.F.; Nadon, N.; Strong, R.; Wood, L.K.; et al. Rapamycin slows aging in mice. Aging Cell 2012, 11, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Feng, Z.; Li, Y.; Wang, Y.; Wertz, K.; Weber, P.; Fu, Y.; Liu, J. Stimulation of gsh synthesis to prevent oxidative stress-induced apoptosis by hydroxytyrosol in human retinal pigment epithelial cells: Activation of Nrf2 and JNK-p62/SQSTM1 pathways. J. Nutr. Biochem. 2012, 23, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Pluquet, O.; Pourtier, A.; Abbadie, C. The unfolded protein response and cellular senescence. A review in the theme: Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. Am. J. Physiol. Cell Physiol. 2015, 308, C415–C425. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Mariño, G.; Levine, B. Autophagy and the integrated stress response. Mol. Cell 2010, 40, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Sommerweiss, D.; Gorski, T.; Richter, S.; Garten, A.; Kiess, W. Oleate rescues INS-1E β-cells from palmitate-induced apoptosis by preventing activation of the unfolded protein response. Biochem. Biophys. Res. Commun. 2013, 441, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Sieber, J.; Lindenmeyer, M.T.; Kampe, K.; Campbell, K.N.; Cohen, C.D.; Hopfer, H.; Mundel, P.; Jehle, A.W. Regulation of podocyte survival and endoplasmic reticulum stress by fatty acids. Am. J. Physiol. Ren. Physiol. 2010, 299, F821–F829. [Google Scholar] [CrossRef] [PubMed]
- Matos, L.; Gouveia, A.M.; Almeida, H. Er stress response in human cellular models of senescence. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Benvenuti, S.; Cramer, R.; Quinn, C.C.; Bruce, J.; Zvelebil, M.; Corless, S.; Bond, J.; Yang, A.; Hockfield, S.; Burlingame, A.L.; et al. Differential proteome analysis of replicative senescence in rat embryo fibroblasts. Mol. Cell. Proteom. 2002, 1, 280–292. [Google Scholar] [CrossRef]
- Dörr, J.R.; Yu, Y.; Milanovic, M.; Beuster, G.; Zasada, C.; Däbritz, J.H.M.; Lisec, J.; Lenze, D.; Gerhardt, A.; Schleicher, K.; et al. Synthetic lethal metabolic targeting of cellular senescence in cancer therapy. Nature 2013, 501, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Panganiban, R.A.M.; Mungunsukh, O.; Day, R.M. X-irradiation induces er stress, apoptosis, and senescence in pulmonary artery endothelial cells. Int. J. Radiat. Biol. 2013, 89, 656–667. [Google Scholar] [CrossRef] [PubMed]
- Green, C.D.; Olson, L.K. Modulation of palmitate-induced endoplasmic reticulum stress and apoptosis in pancreatic β-cells by stearoyl-coa desaturase and elovl6. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E640–E649. [Google Scholar] [CrossRef] [PubMed]
- Svärd, M.; Biterova, E.I.; Bourhis, J.-M.; Guy, J.E. The crystal structure of the human co-chaperone P58 (IPK). PLoS ONE 2011, 6, e22337. [Google Scholar]
- Yan, W.; Frank, C.L.; Korth, M.J.; Sopher, B.L.; Novoa, I.; Ron, D.; Katze, M.G. Control of PERK eIF2α kinase activity by the endoplasmic reticulum stress-induced molecular chaperone p58ipk. Proc. Natl. Acad. Sci. USA 2002, 99, 15920–15925. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, R.L.; Haynes, C.M.; Ron, D. Snapshot: The unfolded protein response. Cell 2010, 140, e590–e592. [Google Scholar] [CrossRef] [PubMed]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cao, J.; Zhong, L. Hydroxytyrosol inhibits pro-inflammatory cytokines, iNOS, and COX-2 expression in human monocytic cells. Naunyn Schmiedeberg’s Arch. Pharmacol. 2009, 379, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Sánchez, J.; Segura-Carretero, A.; Menendez, J.A.; Oliveras-Ferraros, C.; Cerretani, L.; Fernández-Gutiérrez, A. Prediction of extra virgin olive oil varieties through their phenolic profile. Potential cytotoxic activity against human breast cancer cells. J. Agric. Food Chem. 2010, 58, 9942–9955. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Joven, J.; Aragonès, G.; Barrajón-Catalán, E.; Beltrán-Debón, R.; Borrás-Linares, I.; Camps, J.; Corominas-Faja, B.; Cufí, S.; Fernández-Arroyo, S.; et al. Xenohormetic and anti-aging activity of secoiridoid polyphenols present in extra virgin olive oil: A new family of gerosuppressant agents. Cell Cycle 2013, 12, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, N.; Huffman, D.M.; Muzumdar, R.H.; Bartke, A. The critical role of metabolic pathways in aging. Diabetes 2012, 61, 1315–1322. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Partridge, L.; Longo, V.D. Dietary restriction, growth factors and aging: From yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, B.; van der Pluijm, I.; Moorhouse, M.J.; Kosteas, T.; Robinson, A.R.; Suh, Y.; Breit, T.M.; van Steeg, H.; Niedernhofer, L.J.; van Ijcken, W.; et al. Delayed and accelerated aging share common longevity assurance mechanisms. PLoS Genet. 2008, 4, e1000161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garinis, G.A.; van der Horst, G.T.; Vijg, J.; Hoeijmakers, J.H. DNA damage and ageing: New-age ideas for an age-old problem. Nat. Cell Biol. 2008, 10, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Rincon, M.; Rudin, E.; Barzilai, N. The insulin/IGF-1 signaling in mammals and its relevance to human longevity. Exp. Gerontol. 2005, 40, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.; Zarzuelo, A.; Gamez, M.J.; Utrilla, M.P.; Jimenez, J.; Osuna, I. Hypoglycemic activity of olive leaf. Planta Med. 1992, 58, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Al-Azzawie, H.F.; Alhamdani, M.S.S. Hypoglycemic and antioxidant effect of oleuropein in alloxan-diabetic rabbits. Life Sci. 2006, 78, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Hamden, K.; Allouche, N.; Damak, M.; Elfeki, A. Hypoglycemic and antioxidant effects of phenolic extracts and purified hydroxytyrosol from olive mill waste in vitro and in rats. Chem. Biol. Interact. 2009, 180, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Jemai, H.; el Feki, A.; Sayadi, S. Antidiabetic and antioxidant effects of hydroxytyrosol and oleuropein from olive leaves in alloxan-diabetic rats. J. Agric. Food Chem. 2009, 57, 8798–8804. [Google Scholar] [CrossRef] [PubMed]
- De Bock, M.; Derraik, J.G.; Brennan, C.M.; Biggs, J.B.; Morgan, P.E.; Hodgkinson, S.C.; Hofman, P.L.; Cutfield, W.S. Olive (Olea europaea L.) leaf polyphenols improve insulin sensitivity in middle-aged overweight men: A randomized, placebo-controlled, crossover trial. PLoS ONE 2013, 8, e57622. [Google Scholar]
- Johnson, S.C.; Rabinovitch, P.S.; Kaeberlein, M. mTOR is a key modulator of ageing and age-related disease. Nature 2013, 493, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Gonzalez-Angulo, A.M. Targeting the mtor signaling network for cancer therapy. J. Clin. Oncol. 2009, 27, 2278–2287. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A. Targeting PI3K signalling in cancer: Opportunities, challenges and limitations. Nat. Rev. Cancer 2009, 9, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, A.; Majumder, S.; Richardson, A.; Strong, R.; Oddo, S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-β, and Tau: Effects on cognitive impairments. J. Biol. Chem. 2010, 285, 13107–13120. [Google Scholar] [CrossRef] [PubMed]
- Chano, T.; Okabe, H.; Hulette, C.M. RB1CC1 insufficiency causes neuronal atrophy through mTOR signaling alteration and involved in the pathology of Alzheimer’s diseases. Brain Res. 2007, 1168, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Zoncu, R.; Sabatini, D.M.; Efeyan, A. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Don, A.S.; Zheng, X.F. Recent clinical trials of mtor-targeted cancer therapies. Rev. Recent Clin. Trials 2011, 6, 24–35. [Google Scholar] [PubMed]
- Shaw, B.; Lambert, S.; Wong, M.H.; Ralston, J.C.; Stryjecki, C.; Mutch, D.M. Individual saturated and monounsaturated fatty acids trigger distinct transcriptional networks in differentiated 3T3-L1 preadipocytes. J. Nutrigenet. Nutrigenom. 2013, 6, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Khanfar, M.A.; Bardaweel, S.K.; Akl, M.R.; el Sayed, K.A. Olive oil-derived oleocanthal as potent inhibitor of mammalian target of rapamycin: Biological evaluation and molecular modeling studies. Phytother. Res. 2015, 29, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Hekimi, S.; Lapointe, J.; Wen, Y. Taking a “good” look at free radicals in the aging process. Trends Cell Biol. 2011, 21, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.L.; Huertas, J.R.; Manas, M.; Ochoa, J.J.; Battino, M.; Mataix, J. Oxidative stress induced by exercise and dietary fat modulates the coenzyme q and vitamin a balance between plasma and mitochondria. Int. J. Vitam. Nutr. Res. 1999, 69, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Mataix, J.; Ochoa, J.J.; Quiles, J.L. Olive oil and mitochondrial oxidative stress. International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. J. Int. Vitaminol. Nutr. 2006, 76, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Quiles, J.L.; Martinez, E.; Ibanez, S.; Ochoa, J.J.; Martin, Y.; Lopez-Frias, M.; Huertas, J.R.; Mataix, J. Ageing-related tissue-specific alterations in mitochondrial composition and function are modulated by dietary fat type in the rat. J. Bioenerget. Biomembr. 2002, 34, 517–524. [Google Scholar] [CrossRef]
- Ochoa, J.J.; Quiles, J.L.; Ibanez, S.; Martinez, E.; Lopez-Frias, M.; Huertas, J.R.; Mataix, J. Aging-related oxidative stress depends on dietary lipid source in rat postmitotic tissues. J. Bioenerget. Biomembr. 2003, 35, 267–275. [Google Scholar] [CrossRef]
- Barja, G. Rate of generation of oxidative stress-related damage and animal longevity. Free Radic. Biol. Med. 2002, 33, 1167–1172. [Google Scholar] [CrossRef]
- Van der Loo, B.; Labugger, R.; Aebischer, C.P.; Skepper, J.N.; Bachschmid, M.; Spitzer, V.; Kilo, J.; Altwegg, L.; Ullrich, V.; Luscher, T.F. Cardiovascular aging is associated with vitamin E increase. Circulation 2002, 105, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Beckman, K.B.; Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [PubMed]
- Brown-Borg, H.M.; Rakoczy, S.G. Catalase expression in delayed and premature aging mouse models. Exp. Gerontol. 2000, 35, 199–212. [Google Scholar] [CrossRef]
- Bronnikov, G.E.; Kulagina, T.P.; Aripovskii, A.V.; Kramarova, L.I. Correction of mitochondrial enzyme activities in the skeletal muscles of old rats in response to addition of olive oil to the ration. Bull. Exp. Biol. Med. 2015, 159, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Jeck, W.R.; Siebold, A.P.; Sharpless, N.E. Review: A meta-analysis of gwas studies and age-associated diseases. Aging Cell 2012, 11, 727–731. [Google Scholar] [CrossRef] [PubMed]
- Hebert, L.E.; Scherr, P.A.; Bienias, J.L.; Bennett, D.A.; Evans, D.A. State-specific projections through 2025 of Alzheimer disease prevalence. Neurology 2004, 62, 1645. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Solfrizzi, V.; Colacicco, A.M.; D’Introno, A.; Capurso, C.; Torres, F.; del Parigi, A.; Capurso, S.; Capurso, A. Mediterranean diet and cognitive decline. Public Health Nutr. 2004, 7, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; Panza, F.; Capurso, A. The role of diet in cognitive decline. J. Neural Transm. 2003, 110, 95–110. [Google Scholar] [PubMed]
- Scarmeas, N.; Luchsinger, J.A.; Schupf, N.; Brickman, A.M.; Cosentino, S.; Tang, M.X.; Stern, Y. Physical activity, diet, and risk of Alzheimer disease. JAMA 2009, 302, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, G.K.; Keast, R.S.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.H.; Smith, A.B.; Breslin, P.A. Phytochemistry: Ibuprofen-like activity in extra-virgin olive oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Umeno, A.; Takashima, M.; Murotomi, K.; Nakajima, Y.; Koike, T.; Matsuo, T.; Yoshida, Y. Radical-scavenging activity and antioxidative effects of olive leaf components oleuropein and hydroxytyrosol in comparison with homovanillic alcohol. J. Oleo Sci. 2015, 64, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Bellomo, G.; Galli, C. Free radical-scavenging properties of olive oil polyphenols. Biochem. Biophys. Res. Commun. 1998, 247, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Morató, J.; Xicota, L.; Fitó, M.; Farré, M.; Dierssen, M.; de la Torre, R. Potential role of olive oil phenolic compounds in the prevention of neurodegenerative diseases. Molecules 2015, 20, 4655–4680. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J. The amyloid hypothesis for Alzheimer’s disease: A critical reappraisal. J. Neurochem. 2009, 110, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.C.; Liu, R.; Lu, P.; Shapiro, A.B.; Renoir, J.M.; Sharom, F.J.; Reiner, P.B. β-amyloid efflux mediated by p-glycoprotein. J. Neurochem. 2001, 76, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Liu, F.; Gong, C.X.; Alonso Adel, C.; Grundke-Iqbal, I. Mechanisms of Tau-induced neurodegeneration. Acta Neuropathol. 2009, 118, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Abuznait, A.H.; Cain, C.; Ingram, D.; Burk, D.; Kaddoumi, A. Up-regulation of p-glycoprotein reduces intracellular accumulation of beta amyloid: Investigation of p-glycoprotein as a novel therapeutic target for alzheimer’s disease. J. Pharm. Pharmacol. 2011, 63, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J. A hundred years of alzheimer’s disease research. Neuron 2006, 52, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, K.; Nishiura, C.; Mizushima, F.; Minoura, K.; Sumida, M.; Taniguchi, T.; Tomoo, K.; Ishida, T. Linkage-dependent contribution of repeat peptides to self-aggregation of three- or four-repeat microtubule-binding domains in tau protein. FEBS J. 2008, 275, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Mukrasch, M.D.; Biernat, J.; von Bergen, M.; Griesinger, C.; Mandelkow, E.; Zweckstetter, M. Sites of Tau important for aggregation populate β-structure and bind to microtubules and polyanions. J. Biol. Chem. 2005, 280, 24978–24986. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.D.; di Clerico, J.; Li, B.; Corbo, C.P.; Alaniz, M.E.; Grundke-Iqbal, I.; Iqbal, K. Phosphorylation of Tau at Thr212, Thr231, and Ser262 combined causes neurodegeneration. J. Biol. Chem. 2010, 285, 30851–30860. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lee, V.M. Characterization of two vqixxk motifs for tau fibrillization in vitro. Biochemistry 2006, 45, 15692–15701. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sperry, J.B.; Crowe, A.; Trojanowski, J.Q.; Smith, A.B., III; Lee, V.M. Inhibition of Tau fibrillization by oleocanthal via reaction with the amino groups of Tau. J. Neurochem. 2009, 110, 1339–1351. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.C.; Margarucci, L.; Tosco, A.; Riccio, R.; Casapullo, A. New insights on the interaction mechanism between tau protein and oleocanthal, an extra-virgin olive-oil bioactive component. Food Funct. 2011, 2, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.C.; Margarucci, L.; Riccio, R.; Casapullo, A. Modulation of tau protein fibrillization by oleocanthal. J. Nat.Prod. 2012, 75, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Abuznait, A.H.; Qosa, H.; Busnena, B.A.; el Sayed, K.A.; Kaddoumi, A. Olive-oil-derived oleocanthal enhances beta-amyloid clearance as a potential neuroprotective mechanism against Alzheimer’s disease: In vitro and in vivo studies. ACS Chem. Neurosci. 2013, 4, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Qosa, H.; Mohamed, L.A.; Batarseh, Y.S.; Alqahtani, S.; Ibrahim, B.; LeVine, H., III; Keller, J.N.; Kaddoumi, A. Extra-virgin olive oil attenuates amyloid-β and tau pathologies in the brains of tgswdi mice. J. Nutr. Biochem. 2015, 26, 1479–1490. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Xu, F.; Deane, R.; Romanov, G.; Previti, M.L.; Zeigler, K.; Zlokovic, B.V.; van Nostrand, W.E. Early-onset and robust cerebral microvascular accumulation of amyloid β-protein in transgenic mice expressing low levels of a vasculotropic dutch/iowa mutant form of amyloid beta-protein precursor. J. Biol. Chem. 2004, 279, 20296–20306. [Google Scholar] [CrossRef] [PubMed]
- Kline, A. Apolipoprotein e, amyloid-ss clearance and therapeutic opportunities in Alzheimer’s disease. Alzheimer’s Res. Ther. 2012, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Wildsmith, K.R.; Holley, M.; Savage, J.C.; Skerrett, R.; Landreth, G.E. Evidence for impaired amyloid β clearance in Alzheimer’s disease. Alzheimer’s Res. Ther. 2013, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Qosa, H.; Batarseh, Y.S.; Mohyeldin, M.M.; el Sayed, K.A.; Keller, J.N.; Kaddoumi, A. Oleocanthal enhances amyloid-beta clearance from the brains of TgSwDI mice and in vitro across a human blood-brain barrier model. ACS Chem. Neurosci. 2015, 6, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Rodriguez, J.J.; Steardo, L. Astrogliopathology: A central element of neuropsychiatric diseases? Neuroscientist 2014, 20, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Heppner, F.L.; Ransohoff, R.M.; Becher, B. Immune attack: The role of inflammation in Alzheimer disease. Nat. Rev. Neurosci. 2015, 16, 358–372. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Balazs, R.; Soiampornkul, R.; Thangnipon, W.; Cotman, C.W. Interleukin-1β a impairs brain derived neurotrophic factor-induced signal transduction. Neurobiol. Aging 2008, 29, 1380–1393. [Google Scholar] [CrossRef] [PubMed]
- Gruber, R.; Koch, H.; Doll, B.A.; Tegtmeier, F.; Einhorn, T.A.; Hollinger, J.O. Fracture healing in the elderly patient. Exp. Gerontol. 2006, 41, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Moerman, E.J.; Teng, K.; Lipschitz, D.A.; Lecka-Czernik, B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR-γ2 transcription factor and TGF-β/BMP signaling pathways. Aging Cell 2004, 3, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Duque, G. Bone and fat connection in aging bone. Curr. Opin. Rheumatol. 2008, 20, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Mora, R.; Casado-Diaz, A.; de Castro, M.D.; Quesada-Gomez, J.M. Oleuropein enhances osteoblastogenesis and inhibits adipogenesis: The effect on differentiation in stem cells derived from bone marrow. Osteoporos. Int. 2011, 22, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.N.; Galeano-Diaz, T.; Lopez, O.; Fernandez-Bolanos, J.G.; Sanchez, J.; de Miguel, C.; Gil, M.V.; Martin-Vertedor, D. Phenolic compounds and antioxidant capacity of virgin olive oil. Food Chem. 2014, 163, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, S.; Lion, J.M.; Mentaverri, R.; Ricupero, D.A.; Kamel, S.; Romero, J.R.; Chattopadhyay, N. Attenuation of osteoclastogenesis and osteoclast function by apigenin. Biochem. Pharmacol. 2006, 72, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Huang, H.; Li, B.; Wu, D.; Wang, F.; Zheng, X.; Chen, Q.; Wu, B.; Fan, X. Olive oil in the prevention and treatment of osteoporosis after artificial menopause. Clin. Interv. Aging 2014, 9, 2087–2095. [Google Scholar] [CrossRef] [PubMed]
- Shaw, A.C.; Joshi, S.; Greenwood, H.; Panda, A.; Lord, J.M. Aging of the innate immune system. Curr. Opin. Immunol. 2010, 22, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Aldelhay, E.; Pizzatti, L.; Binato, R. Networks establishing hepatopoietic stem cell multipotency and self-renewal. In Advances in Hematopoietic Stem Cell Research; Pelayo, R., Ed.; InTech: Vienna, Austria, 2012; pp. 3–38. [Google Scholar]
- Samet, I. Effect of olive leaf components on the proliferation and viability of hematopoietic stem cells. Asian J. Biomed. Pharm. Sci. 2014, 4, 1–7. [Google Scholar] [CrossRef]
- Cardoso, C.R.; Favoreto, S., Jr.; Oliveira, L.L.; Vancim, J.O.; Barban, G.B.; Ferraz, D.B.; Silva, J.S. Oleic acid modulation of the immune response in wound healing: A new approach for skin repair. Immunobiology 2011, 216, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.H.; Lee, S.J.; Oh, S.Y.; Lee, H.J.; Ryu, J.M.; Han, H.J. Oleic acid enhances the motility of umbilical cord blood derived mesenchymal stem cells through EphB2-dependent F-actin formation. Biochim. Biophys. Acta 2015, 1853, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Gulati, R.; Jevremovic, D.; Peterson, T.E.; Witt, T.A.; Kleppe, L.S.; Mueske, C.S.; Lerman, A.; Vile, R.G.; Simari, R.D. Autologous culture-modified mononuclear cells confer vascular protection after arterial injury. Circulation 2003, 108, 1520–1526. [Google Scholar] [CrossRef] [PubMed]
- Levonen, A.L.; Inkala, M.; Heikura, T.; Jauhiainen, S.; Jyrkkanen, H.K.; Kansanen, E.; Maatta, K.; Romppanen, E.; Turunen, P.; Rutanen, J.; et al. Nrf2 gene transfer induces antioxidant enzymes and suppresses smooth muscle cell growth in vitro and reduces oxidative stress in rabbit aorta in vivo. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Dulak, J.; Loboda, A.; Jozkowicz, A. Effect of heme oxygenase-1 on vascular function and disease. Curr. Opin. Lipidol. 2008, 19, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Rosignoli, P.; Fuccelli, R.; Fabiani, R.; Servili, M.; Morozzi, G. Effect of olive oil phenols on the production of inflammatory mediators in freshly isolated human monocytes. J. Nutr. Biochem. 2013, 24, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Blomberg, B.B. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology 2016, 17, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, M.C.; de Stefano, D.; di Meglio, P.; Irace, C.; Savarese, M.; Sacchi, R.; Cinelli, M.P.; Carnuccio, R. Hydroxytyrosol, a phenolic compound from virgin olive oil, prevents macrophage activation. Naunyn Schmiedeberg’s Arch. Pharmacol. 2005, 371, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Dell’Agli, M.; Fagnani, R.; Galli, G.V.; Maschi, O.; Gilardi, F.; Ballosta, E.; Crestani, M.; Bosisio, E.; de Fabiani, E.; Caruso, D. Olive oil phenols modulate the expression of metalloproteinase 9 in Thp-1 cells by acting on nfkb signalling. J. Agric. Food Chem. 2010, 58, 2246–2252. [Google Scholar] [CrossRef] [PubMed]
- Arango, D.; Diosa-Toro, M.; Rojas-Hernandez, L.S.; Cooperstone, J.L.; Schwartz, S.J.; Mo, X.; Jiang, J.; Schmittgen, T.D.; Doseff, A.I. Dietary apigenin reduces LPS-induced expression of miR-155 restoring immune balance during inflammation. Mol. Nutr. Food Res. 2015, 59, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Dell’Agli, M.; Fagnani, R.; Mitro, N.; Scurati, S.; Masciadri, M.; Mussoni, L.; Galli, G.V.; Bosisio, E.; Crestani, M.; de Fabiani, E.; et al. Minor components of olive oil modulate proatherogenic adhesion molecules involved in endothelial activation. J. Agric. Food Chem. 2006, 54, 3259–3264. [Google Scholar] [CrossRef] [PubMed]
- Zambonin, L.; Caliceti, C.; Vieceli Dalla Sega, F.; Fiorentini, D.; Hrelia, S.; Landi, L.; Prata, C. Dietary phenolic acids act as effective antioxidants in membrane models and in cultured cells, exhibiting proapoptotic effects in leukaemia cells. Oxid. Med.Cell. Longev. 2012, 2012, 839298. [Google Scholar] [CrossRef] [PubMed]
- Meza-Miranda, E.R.; Rangel-Zuniga, O.A.; Marin, C.; Perez-Martinez, P.; Delgado-Lista, J.; Haro, C.; Pena-Orihuela, P.; Jimenez-Morales, A.I.; Malagon, M.M.; Tinahones, F.J.; et al. Virgin olive oil rich in phenolic compounds modulates the expression of atherosclerosis-related genes in vascular endothelium. Eur. J. Nutr. 2015. [Google Scholar] [CrossRef] [PubMed]
- Santos-Gonzalez, M.; Lopez-Miranda, J.; Perez-Jimenez, F.; Navas, P.; Villalba, J.M. Dietary oil modifies the plasma proteome during aging in the rat. Age 2012, 34, 341–358. [Google Scholar] [CrossRef] [PubMed]
- Cunha, T.M.; Verri, W.A., Jr.; Silva, J.S.; Poole, S.; Cunha, F.Q.; Ferreira, S.H. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 1755–1760. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Geng, C.; Jiang, L.; Cao, J.; Yoshimura, H.; Zhong, L. Effects of hydroxytyrosol-20 on carrageenan-induced acute inflammation and hyperalgesia in rats. Phytother. Res. 2009, 23, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Fidalgo, S.; Sanchez de Ibarguen, L.; Cardeno, A.; Alarcon de la Lastra, C. Influence of extra virgin olive oil diet enriched with hydroxytyrosol in a chronic dss colitis model. Eur. J. Nutr. 2012, 51, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Sepodes, B.; Rocha, J.; Direito, R.; Fernandes, A.; Brites, D.; Freitas, M.; Fernandes, E.; Bronze, M.R.; Figueira, M.E. Protective effects of hydroxytyrosol-supplemented refined olive oil in animal models of acute inflammation and rheumatoid arthritis. J. Nutr. Biochem. 2015, 26, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, M.; Barbul, A. Lymphocyte function in wound healing and following injury. Br. J. Surg. 1998, 85, 444–460. [Google Scholar] [CrossRef] [PubMed]
- Peranteau, W.H.; Zhang, L.; Muvarak, N.; Badillo, A.T.; Radu, A.; Zoltick, P.W.; Liechty, K.W. IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J. Investig. Dermatol. 2008, 128, 1852–1860. [Google Scholar] [CrossRef] [PubMed]
- Schwenke, D.C. Aging, menopause, and free radicals. Semin. Reprod. Endocrinol. 1998, 16, 281–308. [Google Scholar] [CrossRef] [PubMed]
- Fitó, M.; Cladellas, M.; de la Torre, R.; Marti, J.; Munoz, D.; Schroder, H.; Alcantara, M.; Pujadas-Bastardes, M.; Marrugat, J.; Lopez-Sabater, M.C.; et al. Anti-inflammatory effect of virgin olive oil in stable coronary disease patients: A randomized, crossover, controlled trial. Eur. J. Clin. Nutr. 2008, 62, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Loued, S.; Berrougui, H.; Componova, P.; Ikhlef, S.; Helal, O.; Khalil, A. Extra-virgin olive oil consumption reduces the age-related decrease in hdl and paraoxonase 1 anti-inflammatory activities. Br. J. Nutr. 2013, 110, 1272–1284. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, Y.M.; Bemudez, B.; Lopez, S.; Abia, R.; Villar, J.; Muriana, F.J. Minor compounds of olive oil have postprandial anti-inflammatory effects. Br. J. Nutr. 2007, 98, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Camargo, A.; Ruano, J.; Fernandez, J.M.; Parnell, L.D.; Jimenez, A.; Santos-Gonzalez, M.; Marin, C.; Perez-Martinez, P.; Uceda, M.; Lopez-Miranda, J.; et al. Gene expression changes in mononuclear cells in patients with metabolic syndrome after acute intake of phenol-rich virgin olive oil. BMC Genom. 2010, 11, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konstantinidou, V.; Covas, M.I.; Munoz-Aguayo, D.; Khymenets, O.; de la Torre, R.; Saez, G.; Tormos Mdel, C.; Toledo, E.; Marti, A.; Ruiz-Gutierrez, V.; et al. In vivo nutrigenomic effects of virgin olive oil polyphenols within the frame of the mediterranean diet: A randomized controlled trial. FASEB J. 2010, 24, 2546–2557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aiello, A.; Guccione, G.D.; Accardi, G.; Caruso, C. What olive oil for healthy ageing? Maturitas 2015, 80, 117–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perri, E.; Raffaelli, A.; Sindona, G. Quantitation of oleuropein in virgin olive oil by ionspray mass spectrometry-selected reaction monitoring. J. Agric. Food Chem. 1999, 47, 4156–4160. [Google Scholar] [CrossRef] [PubMed]
- Brenes, M.; Garcia, A.; Garcia, P.; Rios, J.J.; Garrido, A. Phenolic compounds in spanish olive oils. J. Agric. Food Chem. 1999, 47, 3535–3540. [Google Scholar] [CrossRef] [PubMed]
- Karkoula, E.; Skantzari, A.; Melliou, E.; Magiatis, P. Direct measurement of oleocanthal and oleacein levels in olive oil by quantitative 1H-NMR. Establishment of a new index for the characterization of extra virgin olive oils. J. Agric. Food Chem. 2012, 60, 11696–11703. [Google Scholar] [CrossRef] [PubMed]
- Karkoula, E.; Skantzari, A.; Melliou, E.; Magiatis, P. Quantitative measurement of major secoiridoid derivatives in olive oil using qnmr. Proof of the artificial formation of aldehydic oleuropein and ligstroside aglycon isomers. J. Agric. Food Chem. 2014, 62, 600–607. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández del Río, L.; Gutiérrez-Casado, E.; Varela-López, A.; Villalba, J.M. Olive Oil and the Hallmarks of Aging. Molecules 2016, 21, 163. https://doi.org/10.3390/molecules21020163
Fernández del Río L, Gutiérrez-Casado E, Varela-López A, Villalba JM. Olive Oil and the Hallmarks of Aging. Molecules. 2016; 21(2):163. https://doi.org/10.3390/molecules21020163
Chicago/Turabian StyleFernández del Río, Lucía, Elena Gutiérrez-Casado, Alfonso Varela-López, and José M. Villalba. 2016. "Olive Oil and the Hallmarks of Aging" Molecules 21, no. 2: 163. https://doi.org/10.3390/molecules21020163
APA StyleFernández del Río, L., Gutiérrez-Casado, E., Varela-López, A., & Villalba, J. M. (2016). Olive Oil and the Hallmarks of Aging. Molecules, 21(2), 163. https://doi.org/10.3390/molecules21020163