A Convenient Synthesis of 3,7′-Bisindole Derivatives
Abstract
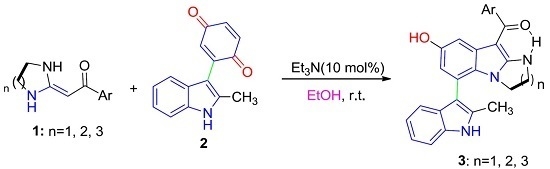
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Information and Materials
3.2. General Procedure for for the Preparation of the 3,7′-Bisindole Derivatives 3a–3w
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fang, E.; Potts, B.C.M.; Faulkner, D.J. 6-Bromotryptamine Derivatives from the Gulf of California Tunicate Didemnum Candidum. J. Nat. Prod. 1991, 54, 564–569. [Google Scholar]
- Ells, R.; Carmeli, H.; Vibrindole, A. A Metabolite of the Marine Bacterium, Vzbrzo Parahaemolytzcus, Isolated from the Mucus of the Boxfish Ostraczon Cubzcus. J. Nat. Prod. 1994, 57, 1587–1590. [Google Scholar]
- Bifulco, G.; Bruno, I.; Riccio, R. Further Brominated Bis- and Tris-Indole Alkaloids from the Deep-Water New Caledonian Marine Sponge Orzna Sp. J. Nat. Prod. 1995, 58, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Garbe, T.R.; Kobayashi, M.; Shimizu, N.; Takesue, N.; Ozawa, M.; Yukawa, H. Indolyl Carboxylic Acids by Condensation of Indoles with α-Keto Acids. J. Nat. Prod. 2000, 63, 596–598. [Google Scholar] [CrossRef] [PubMed]
- Ozkazanc, H. Characterization and Charge Transfer Mechanism of PIN–CdSe Nanocomposites. Polym. Compos. 2015. [Google Scholar] [CrossRef]
- Sazou, D. The dynamical behavior of the electrochemical polymerization of indole on Fe in acetonitrile–water mixtures. Synth. Met. 2002, 130, 45–54. [Google Scholar] [CrossRef]
- Ismail, A.A.; Sanad, S.H.; El-Meligi, A.A. Inhibiting effect of indole and some of its derivatives on corrosion of C-steel in HCl. J. Mater. Sci. Technol. 2000, 16, 397–400. [Google Scholar]
- Dudukcu, M.; Yazici, B.; Erbil, M. The effect of indole on the corrosion behaviour of stainless steel. Mater. Chem Phys. 2004, 87, 138–141. [Google Scholar] [CrossRef]
- Zoraghi, R.; Worrall, L.; See, R.H.; Strangman, W.; Popplewell, W.L.; Gong, H.; Samaai, T.; Swayze, R.D.; Kaur, S.; Vuckovic, M.; et al. Methicillin-resistant Staphylococcus aureus (MRSA) pyruvate kinase as a target for bis-indole alkaloids with antibacterial activities. J. Biol. Chem. 2011, 286, 44716–44725. [Google Scholar] [CrossRef] [PubMed]
- Veale, C.G.L.; Zoraghi, R.; Young, R.M.; Morrison, J.P.; Pretheeban, M.; Lobb, K.A.; Reiner, N.E.; Andersen, R.J.; Davies-Coleman, M.T. Synthetic Analogues of the Marine Bisindole Deoxytopsentin: Potent Selective Inhibitors of MRSA Pyruvate Kinase. J. Nat. Prod. 2015, 78, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Miyake, F.Y.; Yakushijin, K.; Horne, D.A. Synthesis of Marine Sponge Bisindole Alkaloids Dihydrohamacanthins. Org. Lett. 2002, 4, 941–943. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, R.J.; Denny, W.A.; Tercel, M.; Pruijn, F.B.; Ashoorzadeh, A. Nitro seco Analogues of the Duocarmycins Containing Sulfonate Leaving Groups as Hypoxia-Activated Prodrugs for Cancer Therapy. J. Med. Chem. 2012, 55, 2780–2802. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.Y.; Etoh, T.; Hayashi, M.; Komiyama, K.; Kam, T.S. Leucoridines A−D, Cytotoxic Strychnos−Strychnos Bisindole Alkaloids from Leuconotis. J. Nat. Prod. 2010, 73, 1107–1111. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Sun, Q.; Yao, X.; Hong, J.; Lee, C.O.; Sim, C.J.; Im, K.S.; Jung, J.H. Cytotoxic Bisindole Alkaloids from a Marine Sponge Spongosorites sp. J. Nat. Prod. 2005, 68, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.P.; Roy, S. Carbazole Alkaloids III. Prog. Chem. Org. Nat. Prod. 1991, 57, 71–152. [Google Scholar]
- Qu, J.; Fang, L.; Ren, X.D.; Lin, Y.B.; Yu, S.S.; Li, L.; Bao, X.Q.; Zhang, D.; Li, Y.; Ma, S.G. Bisindole Alkaloids with Neural Anti-inflammatory Activity from Gelsemium elegans. J. Nat. Prod. 2013, 76, 2203–2209. [Google Scholar] [CrossRef] [PubMed]
- Mcarthur, K.A.; Mitchell, S.S.; Tsueng, G.; Rheingold, A.; White, D.J.; Grodberg, J.; Lam, K.S.; Potts, B.C.M. Lynamicins A−E, Chlorinated Bisindole Pyrrole Antibiotics from a Novel Marine Actinomycete. J. Nat. Prod. 2008, 71, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, Y.; Hara, M.; Nugroho, A.E.; Sugai, M.; Zaima, K.; Kawahara, N.; Goda, Y.; Awang, K.; Hadi, A.H.A.; Litaudon, M.; et al. Bisnicalaterines B and C, Atropisomeric Bisindole Alkaloids from Hunteria zeylanica, Showing Vasorelaxant Activity. J. Org. Chem. 2010, 75, 4218–4223. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.S.B.; Reddy, V.S.; Sunitha, S. Efficient and Eco-Friendly Process for the Synthesis of Bis(1H-indol-3-yl)methanes using Ionic Liquids. Adv. Synth. Catal. 2003, 345, 349–352. [Google Scholar] [CrossRef]
- Gibbs, T.J.K.; Tomkinson, N.C.O. Aminocatalytic preparation of bisindolylalkanes. Org. Biomol. Chem. 2005, 3, 4043–4045. [Google Scholar] [CrossRef] [PubMed]
- Miyake, F.Y.; Yakushijin, K.; Horne, D.A. A Facile Synthesis of Dragmacidin B and 2,5-Bis(6’-bromo-3’-indolyl)piperazine. Org. Lett. 2000, 2, 3185–3187. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, M.; Basak, R.; Ghosh, N.; Harigaya, Y. Michael reaction of indoles with 3-(2’-nitrovinyl)indole under solvent-free conditions and in solution. An efficient synthesis of 2,2-bis(indolyl)nitroethanes and studies on their reduction. Tetrahedron 2004, 60, 1941–1949. [Google Scholar] [CrossRef]
- Ma, S.; Yu, S. Sc(OTf)3-Catalyzed Indolylation of 1,2-Allenic Ketones: Controlled Highly Selective Synthesis of β-Indolyl-α,β-unsaturetated (E)-Enones and β,β-Bisindolyl Ketones. Org. Lett. 2005, 7, 5063–5065. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Abhilash, K.G.; Vidya, N. Practical Synthesis of Triaryl- and Triheteroarylmethanes by Reaction of Aldehydes and Activated Arenes Promoted by Gold(III) Chloride. Org. Lett. 2005, 7, 5857–5859. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.Q.; Deng, L. Enantioselective Friedel-Crafts Reaction of Indoles with Carbonyl Compounds Catalyzed by Bifunctional Cinchona Alkaloids. Org. Lett. 2006, 8, 4063–4065. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, S.; Kobayashi, S. Carboxylic Acid Catalyzed Three-Component Aza-Friedel-Crafts Reactions in Water for the Synthesis of 3-Substituted Indoles. Org. Lett. 2006, 8, 4939–4942. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, S.; Mari, M.; Piersanti, G.; Spadoni, G. Organocatalyzed coupling of indoles with dehydroalanine esters: Synthesis of bis(indolyl)propanoates and indolacrylates. RSC Adv. 2013, 3, 19135–19143. [Google Scholar] [CrossRef]
- Xu, H.Y.; Zi, Y.; Xu, X.P.; Wang, S.Y.; Ji, S.J. TFA-catalyzed CeN bond activation of enamides with indoles: Efficient synthesis of 3,3-bisindolylpropanoates and other bisindolylalkanes. Tetrahedron 2013, 69, 1600–1605. [Google Scholar] [CrossRef]
- Mari, M.; Tassoni, A.; Lucarini, S.; Fanelli, M.; Piersanti, G.; Spadoni, G. Brønsted Acid Catalyzed Bisindolization of α-Amido Acetals: Synthesis and Anticancer Activity of Bis(indolyl)ethanamino Derivatives. Eur. J. Org. Chem. 2014, 2014, 3822–3830. [Google Scholar] [CrossRef]
- Xie, Z.B.; Sun, D.Z.; Jiang, G.F.; Le, Z.G. Facile Synthesis of Bis(indolyl)methanes Catalyzed by α-Chymotrypsin. Molecules 2014, 19, 19665–19677. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.T.; Wang, M.X. Heterocyclic Ketene Aminals. Heterocycles 1994, 37, 1233–1262. [Google Scholar] [CrossRef]
- Wang, K.M.; Yan, S.J.; Lin, J. Heterocyclic Ketene Aminals: Scaffolds for Heterocycle Molecular Diversity. Eur. J. Org. Chem. 2014, 6, 1129–1145. [Google Scholar] [CrossRef]
- Yang, L.F. Heterocyclic Ketene Aminals. Synlett 2014, 25, 2964–2965. [Google Scholar] [CrossRef]
- Yu, F.; Yan, S.; Hu, L.; Wang, Y.; Lin, J. Cascade Reaction of Isatins with Heterocyclic Ketene Aminals: Synthesis of Imidazopyrroloquinoline Derivatives. Org. Lett. 2011, 13, 4782–4785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.C.; Liu, Z.C.; Yang, R.; Zhang, J.H.; Yan, S.J.; Lin, J. Regioselective construction of 1,3-diazaheterocycle fused [1,2-a][1,8]naphthyridine derivatives via cascade reaction of quinolines with heterocyclic ketene aminals: A joint experimental-computational approach. Org. Biomol. Chem. 2013, 11, 7276–7288. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shao, P.; Wang, S.W.; Kong, W.; Wen, L.R. Four-Component Cascade Heteroannulation of Heterocyclic Ketene Aminals: Synthesis of Functionalized Tetrahydroimidazo[1,2-a]pyridine Derivatives. J. Org. Chem. 2012, 77, 8956–8967. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.R.; Sun, Q.C.; Zhang, H.L.; Li, M. A new rapid multicomponent domino heteroannulation of heterocyclic ketene aminals: Solvent-free regioselective synthesis of functionalized benzo[g]imidazo[1,2-a]quinolinediones. Org. Biomol. Chem. 2013, 11, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Chen, Y.; Liu, L.; He, N.; Lin, J. Three-component solvent-free synthesis of highly substituted bicyclic pyridines containing a ring-junction nitrogen. Green Chem. 2010, 12, 2043–2052. [Google Scholar] [CrossRef]
- Yaqub, M.; Arif, N.; Perveen, R.; Batool1, J.; Riaz, M.T.; Yaseen, M. One-Pot Three Component Cascade Synthesis of Fused Ring Quinazoline-2,4-dione Derivatives Employing Heterocyclic Ketene Aminals as a Versatile Synthone. Asian J. Chem. 2015, 27, 1013–1018. [Google Scholar] [CrossRef]
- Xu, W.Y.; Jia, Y.M.; Yang, J.K.; Huang, Z.T.; Yu, C.Y. Reactions of heterocyclic ketene aminals with 2-[3-oxoisobenzofuran-1(3H)-ylidene]malononitrile: Synthesis of novel polyfunctionalized 1,4-dihydropyridine-fused 1,3-diazaheterocycles. Synlett 2010, 11, 1682–1684. [Google Scholar] [CrossRef]
- Ma, Y.L.; Wang, K.M.; Lin, X.R.; Yan, S.J.; Lin, J. Three-component cascade reaction synthesis of polycyclic 1,4-dihydropyridine derivatives in water. Tetrahedron 2014, 70, 6578–6584. [Google Scholar] [CrossRef]
- Zhu, D.D.; Chen, X.B.; Huang, R.; Yan, S.J.; Lin, J. Three-component solvent-free synthesis of fluorine substituted bicyclic pyridines. Tetrahedron 2015, 71, 2363–2368. [Google Scholar] [CrossRef]
- Chen, N.; Meng, X.; Zhu, F.; Cheng, J.; Shao, X.; Li, Z. Tetrahydroindeno[1′,2′:4,5]pyrrolo[1,2-a] imidazol-5(1H)-ones as Novel Neonicotinoid Insecticides: Reaction Selectivity and Substituent Effects on the Activity Level. J. Agric. Food Chem. 2015, 63, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B.; Liu, Z.C.; Yang, L.F.; Yan, S.J.; Lin, J. A Three-Component Catalyst-Free Approach to Regioselective Synthesis of Dual Highly Functionalized Fused Pyrrole Derivatives in Water-Ethanol Media: Thermodynamics versus Kinetics. ACS Sustain. Chem. Eng. 2014, 2, 1155–1163. [Google Scholar] [CrossRef]
- Shao, X.; Fu, H.; Xu, X.; Xu, X.; Liu, Z.; Li, Z.; Qian, X. Divalent and Oxabridged Neonicotinoids Constructed by Dialdehydes and Nitromethylene Analogues of Imidacloprid: Design, Synthesis, Crystal Structure, and Insecticidal Activities. J. Agric. Food Chem. 2010, 58, 2696–2702. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.B.; Liu, Z.C.; Lin, X.R.; Huang, R.; Yan, S.J.; Lin, J. Highly Diastereoselective Convergent Synthesis of Polycyclic Pyrroles with Consecutive Quaternary Stereocenters: Cascade Construction of Multiple C–C and C–Hetero Bonds. ACS Sustain. Chem. Eng. 2014, 2, 2391–2398. [Google Scholar] [CrossRef]
- Chen, X.B.; Wang, X.Y.; Zhu, D.D.; Yan, S.J.; Lin, J. Three-component domino reaction synthesis of highly functionalized bicyclic pyrrole derivatives. Tetrahedron 2014, 70, 1047–1054. [Google Scholar]
- Yu, F.C.; Huang, R.; Ni, X.C.; Fan, J.; Yan, S.J.; Lin, J. Three-component stereoselective synthesis of spirooxindole derivatives. Green Chem. 2013, 15, 453–462. [Google Scholar] [CrossRef]
- Chen, X.B.; Liu, X.M.; Huang, R.; Yan, S.J.; Lin, J. Three-Component Synthesis of Indanone-Fused Spirooxindole Derivatives. Eur. J. Org. Chem. 2013, 2013, 4607–4613. [Google Scholar] [CrossRef]
- Yang, L.J.; Yan, S.J.; Chen, W.; Lin, J. A Facile Route to 1,3-Diazaheterocycle-Fused [1,2-a]Indole Derivatives via Acetic Acid Catalyzed Cyclocondensation Reactions. Synthesis 2010, 20, 3536–3544. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, Z.C.; Qu, W.W.; Yang, R.; Lin, X.R.; Yan, S.J.; Lin, J. An environmentally benign, mild, and catalyst-free reaction of quinones with heterocyclic ketene aminals in ethanol: Site-selective synthesis of rarely fused [1,2-a]indolone derivatives via an unexpected anti-Nenitzescu strategy. Green Chem. 2014, 16, 4359–4370. [Google Scholar] [CrossRef]
- Yu, F.C.; Hao, X.P.; Lin, X.R.; Yan, S.J.; Lin, J. Synthesis of fused polyhalogeno-7a-hydroxy-[1,2-a]indol -5-one derivatives. Tetrahedron 2015, 71, 4084–4089. [Google Scholar] [CrossRef]
- Huang, Z.T.; Wang, M.X. A New Route to 3H-1,5-Benzodiazepines and Heterocylic Ketene Aminals from Benzoyl Substituted Ketene Dithioacetals and Diamines. Synthesis 1992, 12, 1273–1276. [Google Scholar] [CrossRef]
- Li, Z.J.; Charles, D. The Synthesis of fluoroheterocyclic ketene aminals. Synth. Commun. 2001, 31, 527–533. [Google Scholar] [CrossRef]
- Zhang, H.B.; Liu, L.; Chen, Y.J.; Wang, D.; Li, C.J. “On Water”-Promoted Direct Coupling of Indoles with 1,4-Benzoquinones without Catalyst. Eur. J. Org. Chem. 2006, 2006, 869–873. [Google Scholar] [CrossRef]
- Niu, F.; Liu, C.C.; Cui, Z.M.; Zhai, J.; Jiang, L.; Song, W.G. Promotion of organic reactions by interfacial hydrogen bonds on hydroxyl group rich nano-solids. Chem. Commun. 2008, 24, 2803–2805. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 3a–3w are available from the authors.



| Entry | Solvent | Catalyst | t (°C) | Time (h) | Yield (%) b |
|---|---|---|---|---|---|
| 1 | EtOH | − | rt | 12 | trace |
| 2 | EtOH | HOAc | rt | 12 | 45 |
| 3 | EtOH | Et3N | rt | 12 | 91 |
| 4 | EtOH | Na2CO3 | rt | 12 | 67 |
| 5 | EtOH | K2CO3 | rt | 12 | 66 |
| 6 | EtOH | EtONa | rt | 12 | 25 |
| 7 | EtOH | DBU | rt | 12 | trace |
| 8 | CH2Cl2 | Et3N | rt | 12 | 78 |
| 9 | MeCN | Et3N | rt | 12 | 81 |
| 10 | tetrahydrofuran | Et3N | rt | 12 | 75 |
| 11 | toluene | Et3N | rt | 12 | 68 |
| 12 | MeOH | Et3N | rt | 12 | 72 |
| 13 | H2O | Et3N | rt | 12 | 30 |
| 14 | EtOH | Et3N | 40 | 18 | 67 |
| 15 | EtOH | Et3N | reflux | 24 | 45 |
| Entry | n | R1 | R2 | R3 | R4 | R5 | 3 | Yield (%) b |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | MeO | H | H | Me | H | 3a | 91 |
| 2 | 2 | Me | H | H | Me | H | 3b | 88 |
| 3 | 2 | H | H | H | Me | H | 3c | 82 |
| 4 | 2 | Cl | H | H | Me | H | 3d | 89 |
| 5 | 2 | H | Cl | H | Me | H | 3e | 86 |
| 6 | 2 | F | H | H | Me | H | 3f | 89 |
| 7 | 3 | MeO | H | H | Me | H | 3g | 87 |
| 8 | 3 | Me | H | H | Me | H | 3h | 84 |
| 9 | 3 | H | H | H | Me | H | 3i | 83 |
| 10 | 3 | Cl | H | H | Me | H | 3j | 87 |
| 11 | 3 | H | Cl | H | Me | H | 3k | 81 |
| 12 | 3 | F | H | H | Me | H | 3l | 83 |
| 13 | 1 | MeO | H | H | Me | H | 3m | 82 |
| 14 | 1 | Me | H | H | Me | H | 3n | 75 |
| 15 | 1 | H | H | H | Me | H | 3o | 73 |
| 16 | 1 | Cl | H | H | Me | H | 3p | 75 |
| 17 | 1 | H | Cl | H | Me | H | 3q | 72 |
| 18 | 1 | F | H | H | Me | H | 3r | 77 |
| 19 | 2 | MeO | H | Me | Me | H | 3s | 87 |
| 20 | 2 | F | H | Me | Me | H | 3t | 70 |
| 21 | 2 | MeO | H | H | Ph | H | 3u | 85 |
| 22 | 2 | F | H | H | Ph | H | 3v | 75 |
| 23 | 2 | MeO | H | H | H | MeO | 3w | 65 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, T.; Zhu, H.-Y.; Luo, D.-Y.; Yan, S.-J.; Lin, J. A Convenient Synthesis of 3,7′-Bisindole Derivatives. Molecules 2016, 21, 638. https://doi.org/10.3390/molecules21050638
Liu T, Zhu H-Y, Luo D-Y, Yan S-J, Lin J. A Convenient Synthesis of 3,7′-Bisindole Derivatives. Molecules. 2016; 21(5):638. https://doi.org/10.3390/molecules21050638
Chicago/Turabian StyleLiu, Teng, Hong-You Zhu, Da-Yun Luo, Sheng-Jiao Yan, and Jun Lin. 2016. "A Convenient Synthesis of 3,7′-Bisindole Derivatives" Molecules 21, no. 5: 638. https://doi.org/10.3390/molecules21050638
APA StyleLiu, T., Zhu, H. -Y., Luo, D. -Y., Yan, S. -J., & Lin, J. (2016). A Convenient Synthesis of 3,7′-Bisindole Derivatives. Molecules, 21(5), 638. https://doi.org/10.3390/molecules21050638