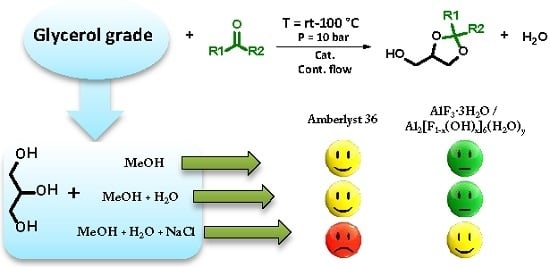
Towards a Rational Design of a Continuous-Flow Method for the Acetalization of Crude Glycerol: Scope and Limitations of Commercial Amberlyst 36 and AlF3·3H2O as Model Catalysts
Abstract
:1. Introduction
2. Results
2.1. The CF-Acetalization of Glycerol with Acetone
2.2. AlF3·3H2O for the Acetalization of Glyc5 and Glyc6: Reaction Productivity
2.3. The CF-Acetalization of Glycerol with 2-Butanone
2.4. Characterization of AlF3·3H2O
3. Discussion
3.1. The Catalyst
3.2. The Continuous-Flow (CF) Conditions
3.3. 2-Butanone
4. Experimental Section
4.1. General
4.2. CF-Apparatus
Safety Warning
4.3. General Procedure for the CF Acetalization Reactions of Glycerol
4.3.1. Preparation of Reactants
4.3.2. Reaction Procedure
4.3.3. System Cleaning and Reuse of the Reactor
4.4. Aluminium and Fluoride Contents by ICP-OES and Ionic Chromatographic Analyses
4.5. Isolation and Characterization of Products
4.5.1. (2,2-dimethyl-1,3-dioxolan-4-yl)methanol (Solketal)
4.5.2. (2-Ethyl-2-methyl-1,3-dioxolan-4-yl)methanol (1c)
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Pagliaro, M.; Rossi, M. The Future of Glycerol; The Royal Society of Chemistry: Cambridge, UK, 2010. [Google Scholar]
- Behr, A.; Eilting, J.; Irawadi, K.; Leschinski, J.; Lindner, F. Improved utilisation of renewable resources: New important derivatives of glycerol. Green Chem. 2008, 10, 13–30. [Google Scholar] [CrossRef]
- Nadir, N. Better Chemistry, Better Biofuels? The Glycerol Glut, Solketal, and Other Floating Ideas. Available online: http://www.theenergycollective.com/nnadir/361711/better-chemistry-better-biofuels- glycerol-glut-solketal-and-other-floating-ideas (accessed on 15 January 2016).
- Burczyk, B.; Piasecki, A.; Weclas, L. Chemical structure and surface activity. 10. The effect of hydroxyl group configuration on the adsorption of 2-alkyl-4-(hydroxymethyl)-1,3-dioxolanes and 2-alkyl-5-hydroxy-1,3-dioxanes at the aqueous solution-air interface. J. Phys. Chem. 1985, 89, 1032–1035. [Google Scholar] [CrossRef]
- Vol’eva, V.B.; Belostotskaya, I.S.; Malkova, A.V.; Komossarova, N.L.; Kurkovskaya, L.N.; Usachev, S.V.; Makarov, G.G. New approach to the synthesis of 1,3-dioxolanes. Russ. J. Org. Chem. 2012, 48, 638–641. [Google Scholar] [CrossRef]
- Dıaz-Alvarez, A.E.; Francos, J.; Lastra-Barreira, B.; Crochet, P.; Cadierno, V. Glycerol and derived solvents: New sustainable reaction media for organic synthesis. Chem. Commun. 2011, 47, 6208–6227. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, V.R.; Velty, A.; Santos, L.L.; Leyva-Pérez, A.; Sabater, M.J.; Iborra, S.; Corma, A. Gold catalysts and solid catalysts for biomass transformations: Valorization of glycerol and glycerol–water mixtures through formation of cyclic acetals. J. Catal. 2010, 271, 351–357. [Google Scholar] [CrossRef]
- Garcıa, E.; Laca, M.; Perez, E.; Garrido, A.; Peinado, J. New Class of Acetal Derived from Glycerin as a Biodiesel Fuel Component. Energ. Fuels 2008, 22, 4274–4280. [Google Scholar] [CrossRef]
- Rahmat, N.; Abdullah, A.Z.; Mohamed, A.R. Recent progress on innovative and potential technologies for glycerol transformation into fuel additives: A critical review. Renew. Sustain. Energ. Rev. 2010, 14, 987–1000. [Google Scholar] [CrossRef]
- Silva, P.H.; Gonçalves, V.L.; Mota, C.J. Glycerol acetals as anti-freezing additives for biodiesel. Bioresource Technol. 2010, 101, 6225–6229. [Google Scholar] [CrossRef] [PubMed]
- Delfort, B.; Durand, I.; Jaecker, A.; Lacome, T.; Montagne, X.; Paille, F. Diesel Fuel Compounds Containing Glycerol Acetals. U.S. Patent 6,890,364 (B2), 10 May 2005. [Google Scholar]
- Selva, M.; Benedet, V.; Fabris, M. Selective catalytic etherification of glycerol formal and solketal with dialkyl carbonates and K2CO3. Green Chem. 2012, 14, 188–200. [Google Scholar] [CrossRef]
- Selva, M.; Guidi, S.; Noè, M. Upgrading of glycerol acetals by thermal catalyst-free transesterification of dialkyl carbonates under continuous-flow conditions. Green Chem. 2015, 17, 1008–1023. [Google Scholar] [CrossRef]
- Meskens, F.A.J. Methods for the Preparation of Acetals from Alcohols or Oxiranes and Carbonyl Compounds. Synthesis 1981, 1981, 501–522. [Google Scholar] [CrossRef]
- Kocienski, P.J. Protecting Groups; G.T. Verlag: Stuttgart, Germany, 2005. [Google Scholar]
- Michael, B.S.; March, J. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure, 6th ed.; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Deutsch, J.; Martin, A.; Lieske, H. Investigations on heterogeneously catalysed condensations of glycerol to cyclic acetals. J. Catal. 2007, 245, 428–435. [Google Scholar] [CrossRef]
- Kaufhold, M.; El-Chahawi, M. Process for Preparing Acetaldehyde Diethyl Acetal. U.S. Patent 5,527,969, 18 June 1996. [Google Scholar]
- Nair, G.S.; Adrijanto, E.; Alsalme, A.; Kozhevnikov, I.V.; Cooke, D.J.; Brown, D.R.; Shiju, N.R. Glycerol utilization: Solvent-free acetalisation over niobia catalysts. Catal. Sci. Technol. 2012, 2, 1173–1179. [Google Scholar] [CrossRef]
- Yamamoto, H.; Ishihara, K. Acid Catalysis in Modern Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Climent, M.J.; Corma, A.; Velty, A. Synthesis of hyacinth, vanilla, and blossom orange fragrances: The benefit of using zeolites and delaminated zeolites as catalysts. Appl. Catal. A Gen. 2004, 263, 155–161. [Google Scholar] [CrossRef]
- Gonzalez-Arellano, C.; Arancon, R.A.D.; Luque, R. Al-SBA-15 catalysed cross-esterification and acetalisation of biomass-derived platform chemicals. Green Chem. 2014, 16, 4985–4993. [Google Scholar] [CrossRef]
- Wang, B.; Shen, Y.; Sun, J.; Xu, F.; Sun, R. Conversion of platform chemical glycerol to cyclic acetals promoted by acidic ionic liquids. RSC Adv. 2014, 4, 18917–18923. [Google Scholar] [CrossRef]
- Selva, M.; Gottardo, M.; Perosa, A. Upgrade of Biomass-Derived Levulinic Acid via Ru/C-Catalyzed Hydrogenation to γ-Valerolactone in Aqueous–Organic–Ionic Liquids Multiphase Systems. ACS Sustain. Chem. Eng. 2013, 1, 180–189. [Google Scholar] [CrossRef] [Green Version]
- Stanley, J.N.G.; Selva, M.; Masters, A.F.; Maschmeyer, T.; Perosa, A. Reactions of p-coumaryl alcohol model compounds with dimethyl carbonate. Towards the upgrading of lignin building blocks. Green Chem. 2013, 15, 3195–3204. [Google Scholar] [CrossRef]
- Yang, F.; Hanna, M.A.; Sun, R. Value-added uses for crude glycerol—a byproduct of biodiesel production. Biotechnol. Biofuels 2012, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, J.S.; Walker, A.J.; Wood, M.A. Continuous Reactor Technology for Ketal Formation: An Improved Synthesis of Solketal. Org. Proc. Res. Dev. 2001, 5, 630–635. [Google Scholar] [CrossRef]
- Monbaliu, J.-C.M.; Winter, M.; Chevalier, B.; Schmidt, F.; Jang, Y.; Hoogendoorn, R.; Kousemaker, M.A.; Stevens, C.V. Effective production of the biodiesel additive STBE by a continuous flow process. Bioresource Technol. 2011, 102, 9304–9307. [Google Scholar] [CrossRef] [PubMed]
- Nanda, M.R.; Yuan, Z.; Qiu, W.; Ghaziaskar, H.S.; Poirier, M.-A.; Xu, C. A new continuous-flow process for catalytic conversion of glycerol to oxygenated fuel additive: Catalyst screening. Appl. Energ. 2014, 123, 75–81. [Google Scholar] [CrossRef]
- Nanda, M.R.; Yuana, Z.; Qinb, W.; Ghaziaskarc, H.S.; Poirierd, M.; Xu, C. Catalytic conversion of glycerol to oxygenated fuel additive in a continuous flow reactor: Process optimization. Fuel 2014, 128, 113–119. [Google Scholar] [CrossRef]
- Shirani, M.; Ghaziaskar, H.S.; Xu, C. Optimization of glycerol ketalization to produce solketal as biodiesel additive in a continuous reactor with subcritical acetone using Purolite® PD206 as catalyst. Fuel Proc. Technol. 2014, 124, 206–211. [Google Scholar] [CrossRef]
- Eltanany, G.; Rüdiger, S.; Kemnitz, E. Supported high surface AlF3: A very strong solid Lewis acid for catalytic applications. J. Mater. Chem. 2008, 18, 2268–2275. [Google Scholar] [CrossRef]
- Tressaud, A. Functionalized Inorganic Fluorides, Synthesis, Characterization and Properties of Nanostructured Solids; John Wiley & Sons, Ltd: Chichester, UK, 2010. [Google Scholar]
- Coman, S.M.; Wuttke, S.; Vimont, A.; Daturi, M.; Kemnitz, E. Catalytic Performance of Nanoscopic, Aluminium Trifluoride-Based Catalysts in the Synthesis of (all-rac)-α-Tocopherol. Adv. Synth. Catal. 2008, 350, 2517–2524. [Google Scholar] [CrossRef]
- Busca, G. Heterogeneous Catalytic Materials, Sold State Chemistry, Surface Chemistry, and Catalytic Behavior; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Corma, A.; Garcıa, H. Lewis Acids: From Conventional Homogeneous to Green Homogeneous and Heterogeneous Catalysis. Chem. Rev. 2003, 103, 4307–4366. [Google Scholar] [CrossRef] [PubMed]
- Harley, A.D.; Puga, J. Aluminum Trifluoride Catalyst for Production of Diaryl Carbonates. U.S. Patent 5252771 (A), 12 October 1993. [Google Scholar]
- MSDS. Available online: http://www.sigmaaldrich.com/ (accessed on 15 January 2016).
- Selva, M.; Guidi, S.; Perosa, A.; Signoretto, M.; Licence, P.; Maschmeyer, T. Continuous-flow alkene metathesis: The model reaction of 1-octene catalyzed by Re2O7/γ-Al2O3 with supercritical CO2 as a carrier. Green Chem. 2012, 14, 2727–2737. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Xiao, G.; Varma, A. A Universal Procedure for Crude Glycerol Purification from Different Feedstocks in Biodiesel Production: Experimental and Simulation Study. Ind. Eng. Chem. Res. 2013, 52, 14291–14296. [Google Scholar] [CrossRef]
- Hu, S.; Luo, X.; Wan, C.; Li, Y. Characterization of Crude Glycerol from Biodiesel Plants. J. Agric. Food Chem. 2012, 60, 5915–5921. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.C.; He, B.B. Characterization of crude glycerol from biodiesel production from multiple feedstocks. Appl. Eng. Agric. 2006, 22, 261–265. [Google Scholar] [CrossRef]
- When necessary, pumps were set at the lowest pressure (~2 bar) allowing the transfer of the reaction mixture through the catalytic bed. Reactants were forced to flow from bottom to top of the reactor to rule out any gravity-driven pathway.
- WHSV is defined as the weight of feed flowing per unit weight of the catalyst per hour; see, Harriott, P. Ideal Reactors. In Chemical Reactor Design; Marcel Dekker, Inc.: New York, NY, USA, 2003; Chapter 3. [Google Scholar]
- In the repeated tests carried out under the same conditions, values of conversion and amount of products (determined by GC/MS) differed by less than 5% from one reaction to another.
- Vicente, G.; Melero, J.A.; Morales, G.; Paniagua, M.; Martin, E. Acetalisation of bio-glycerol with acetone to produce solketal over sulfonic mesostructured silicas. Green Chem. 2010, 12, 899–907. [Google Scholar] [CrossRef]
- Estruga, M.; Meng, F.; Li, L.; Chen, L.; Li, X.; Jin, S. Large-scale solution synthesis of α-AlF3·3H2O nanorods under low supersaturation conditions and their conversion to porous β-AlF3 nanorods. J. Mater. Chem. 2012, 22, 20991–20997. [Google Scholar] [CrossRef]
- Similar data (Al and F contents) were not gathered for the reaction of Glyc5 because of analytical interferences of Na+ and Cl− ions present in the reactant mixture.
- It should be noted that the assignment of structures of diastereoisomers 1c and 1c′ was complicated by a partial overlap of signals in botH-NMR and GC/MS spectra: This drawback was already reported for the characterization of the same compounds and of other glycerol acetals. See, for example, reference [13] and Fadnavis, N.W.; Reddipalli, G.S.; Ramakrishna, G.; Mishra, M.K.; Sheelu, G. Highly Regioselective Preparation of 1,3-Dioxolane-4-methanol Derivatives from Glycerol Using Phosphomolybdic Acid. Synthesis 2009, 4, 557–560. [Google Scholar] [CrossRef]
- Scholz, G.; Brehme, S.; Konig, R.; Heidemann, D.; Kemnitz, E. Crystalline Aluminum Hydroxide Fluorides AlFx(OH)3−x·H2O: Structural Insights from 1H and 2H Solid State NMR and Vibrational Spectroscopy. J. Phys. Chem. C 2010, 114, 10535–10543. [Google Scholar] [CrossRef]
- Chupas, P.J.; Corbin, D.R.; Rao, V.N.M.; Hanson, J.C.; Grey, C.P. A Combined Solid-State NMR and Diffraction Study of the Structures and Acidity of Fluorinated Aluminas: Implications for Catalysis. J. Phys. Chem. B 2003, 107, 8327–8336. [Google Scholar] [CrossRef]
- Rosenberg, P.E. Stability Relations of Aluminum Hydroxy-Fluoride Hydrate, A Ralstonite-like Mineral, in the System AlF3–Al2O3–H2O–HF. Can. Mineral. 2006, 44, 125–134. [Google Scholar]
- Weingarten, R.; Tompsett, G.A.; Conner, W.C., Jr.; Huber, G.W. Design of solid acid catalysts for aqueous-phase dehydration of carbohydrates: The role of Lewis and Brønsted acid sites. J. Catal. 2011, 279, 174–182. [Google Scholar] [CrossRef]
- Hess, A.; Kemnitz, E. Characterization of Catalytically Active Sites on Aluminum Oxides, Hydroxyfluorides, and Fluorides in Correlation with Their Catalytic Behavior. J. Catal. 1994, 149, 449–463. [Google Scholar] [CrossRef]
- Francke, L.; Durand, E.; Demourgues, A.; Vimont, A.; Daturi, M.; Tressaud, A. Synthesis and characterization of Al3+, Cr3+, Fe3+ and Ga3+ hydroxyfluorides: Correlations between structural features, thermal stability and acidic properties. J. Mater. Chem. 2003, 13, 2330–2340. [Google Scholar] [CrossRef]
- Choi, A.L.; Sun, G.; Zhang, Y.; Grandjean, P. Developmental Fluoride Neurotoxicity: A Systematic Review and Meta-Analysis. Environ. Health Perspect. 2012, 120, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- National Primary Drinking Water Regulations. Available online: http://water.epa.gov/drink/contaminants/ (accessed on 15 January 2016).
- Pure α-AlF3 is inactive for the acetalization reaction (Scheme 5). This phase, either pure on in three hydrate form (α-AlF3·3H2O), has been reported as a poor Lewis acid system (see reference [17] and: Murwani, I.K.; Scheurell, K.; Kemnitz, E. Liquid phase oxidation of ethylbenzene on pure and metal doped HS-AlF3. Cat. Commun. 2008, 10, 227–231. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Maki, K.; Matsumura, Y.; Kamegawa, T.; Mori, K.; Yamashita, H. Hydrophobic Modification of a Mesoporous Silica Surface Using a Fluorine-Containing Silylation Agent and Its Application as an Advantageous Host Material for the TiO2 Photocatalyst. J. Phys. Chem. C 2009, 113, 1552–1559. [Google Scholar] [CrossRef]
- Agirre, I.; Garcıa, I.; Requies, J.; Barrio, V.L.; Guemez, M.B.; Cambra, J.F.; Arias, P.L. Glycerol acetals, kinetic study of the reaction between glycerol and formaldehyde. Biomass Bioenerg. 2011, 35, 3636–3642. [Google Scholar] [CrossRef]
- Sample Availability: Samples of all compounds and catalysts are available from the authors.











| Entry | Glycerol: Additives, Molar Ratio b (wt %, Composition) | Reactant Label | |||
|---|---|---|---|---|---|
| Glycerol a | MeOH | H2O | NaCl | ||
| 1 | (100) | none | none | none | Glyc1 |
| 2 | 1 (70) | 1.2 (30) | none | none | Glyc2 c |
| 3 | 1 (68) | 1.2 (28) | 0.3 (4) | none | Glyc3 c |
| 4 | 1 (53) | 1.2 (22) | 2.4 (25) | none | Glyc4 c |
| 5 | 1 (49.5) | 1.2(22) | 2.4 (24) | 0.08(2.5) | Glyc5 c |
| 6 | 1 (44.3) | 0.3 (4.4) | 5.6 (49) | 0.08 (2.3) | Glyc6 c |
| Entry | Reactant | AlF3·3H2O (g) | Q Ratio (mol:mol) b | WHSV | Conv.’n (%) c | Productivity (P) (g of (1b + 1b′)/gcat·h) |
|---|---|---|---|---|---|---|
| 1 | Glyc5 | 0.67 | 4 | 2 | 78 | 2.2 |
| 2 | 4 | 4 | 67 | 3.8 | ||
| 3 | 4 | 6 | 65 | 5.6 | ||
| 4 | 4.2 | 4 | 2 | 75 | 2.2 | |
| 5 | Glyc6 | 0.67 | 4 | 2 | 30 | 0.86 |
| 6 | 8 | 2 | 60 | 1.7 | ||
| 7 | 8 | 4 | 71 | 4.0 | ||
| 8 | 4.2 | 8 | 2 | 54 | 1.6 |
| Entry | Reactant | Q Ratio (mol:mol) | WSHV | Conv.’n (%) b | Productivity (P) (g of (1c + 1c′)/gcat h) |
|---|---|---|---|---|---|
| 1 | Glyc1 | 60 | 2 | 85 | 2.7 |
| 2 | Glyc6 | 4 | 2 | 45 | 1.3 |
| Entry | Sample | Label | Time-on-Stream (h) a |
|---|---|---|---|
| 1 | AlF3·3H2O Fresh | AF | - |
| 2 | Calcined AlF3·3H2O b | AFc | - |
| 3 | AlF3·3H2O after use for Glyc1-4 | AF1 | 30 |
| 4 | AlF3·3H2O after use for Glyc5-6 | AF2 | 90 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guidi, S.; Noè, M.; Riello, P.; Perosa, A.; Selva, M. Towards a Rational Design of a Continuous-Flow Method for the Acetalization of Crude Glycerol: Scope and Limitations of Commercial Amberlyst 36 and AlF3·3H2O as Model Catalysts. Molecules 2016, 21, 657. https://doi.org/10.3390/molecules21050657
Guidi S, Noè M, Riello P, Perosa A, Selva M. Towards a Rational Design of a Continuous-Flow Method for the Acetalization of Crude Glycerol: Scope and Limitations of Commercial Amberlyst 36 and AlF3·3H2O as Model Catalysts. Molecules. 2016; 21(5):657. https://doi.org/10.3390/molecules21050657
Chicago/Turabian StyleGuidi, Sandro, Marco Noè, Pietro Riello, Alvise Perosa, and Maurizio Selva. 2016. "Towards a Rational Design of a Continuous-Flow Method for the Acetalization of Crude Glycerol: Scope and Limitations of Commercial Amberlyst 36 and AlF3·3H2O as Model Catalysts" Molecules 21, no. 5: 657. https://doi.org/10.3390/molecules21050657
APA StyleGuidi, S., Noè, M., Riello, P., Perosa, A., & Selva, M. (2016). Towards a Rational Design of a Continuous-Flow Method for the Acetalization of Crude Glycerol: Scope and Limitations of Commercial Amberlyst 36 and AlF3·3H2O as Model Catalysts. Molecules, 21(5), 657. https://doi.org/10.3390/molecules21050657