Inhalable Antitubercular Therapy Mediated by Locust Bean Gum Microparticles
Abstract
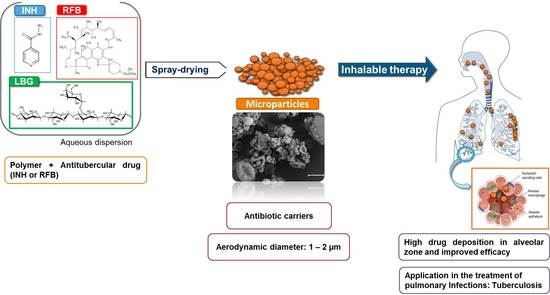
:1. Introduction
2. Results and Discussion
2.1. Preparation of Locust Bean Gum (LBG) Microparticles by Spray-Drying
2.2. Association of Drugs and Characterisation of Microparticles
2.3. Cristallinity of LBG-Based Microparticles
2.4. In Vitro Drug Release
2.5. Cytotoxic Evaluation
2.5.1. Evaluation of Metabolic Activity
2.5.2. Evaluation of Cell Membrane Integrity
2.6. Preliminary Evaluation of Macrophage Ability to Uptake LBG Microparticles
3. Experimental Section
3.1. Materials
3.2. Cell Lines
3.3. Preparation of Locust Bean Gum Microparticles by Spray-Drying
3.4. Characterisation of Microparticles
3.5. Determination of Drug Association
3.6. Crystallinity of Dry Powders
3.7. In Vitro Drug Release
3.8. In Vitro Biocompatibility Study
3.8.1. Evaluation of Metabolic Activity
3.8.2. Determination of Cell Membrane Integrity
3.9. Preliminary Evaluation of Macrophage Ability to Uptake LBG Microparticles
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fogel, N. Tuberculosis: A disease without boundaries. Tuberculosis 2015, 95, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Zumla, A.; Chakaya, J.; Centis, R.; D’Ambrosio, L.; Mwaba, P.; Bates, M.; Kapata, N.; Nyirenda, T.; Chanda, D.; Mfinanga, S.; et al. Tuberculosis treatment and management—An update on treatment regimens, trials, new drugs, and adjunct therapies. Lancet Respir. Med. 2015, 3, 220–234. [Google Scholar] [CrossRef]
- Pham, D.D.; Fattal, E.; Tsapis, N. Pulmonary drug delivery systems for tuberculosis treatment. Int. J. Pharm. 2015, 478, 517–529. [Google Scholar] [CrossRef]
- Hoppentocht, M.; Hagedoorn, P.; Frijlink, H.W.; de Boer, A.H. Developments and strategies for inhaled antibiotic drugs in tuberculosis therapy: A critical evaluation. Eur. J. Pharm. Biopharm. 2014, 86, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.; Shah, V.; Chivate, N. Pulmonary drug delivery: A promising approach. J. Appl. Pharm. Sci. 2012, 2, 33–37. [Google Scholar]
- Pacheco, P.; White, D.; Sulchek, T. Effects of microparticle size and Fc density on macrophage phagocytosis. PLoS ONE 2013, 8, e60989. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, F.; Rivas, I.P.; Khan, M.A.; Torres Suárez, A.I. Targeting to macrophages: Role of physicochemical properties of particulate carriers—Liposomes and microspheres—On the phagocytosis by macrophages. J. Control. Release 2002, 79, 29–40. [Google Scholar] [CrossRef]
- Chow, A.H.; Tong, H.H.; Chattopadhyay, P.; Shekunov, B.Y. Particle engineering for pulmonary drug delivery. Pharm. Res. 2007, 24, 411–437. [Google Scholar] [CrossRef] [PubMed]
- Malafaya, P.; Silva, G.; Reis, R. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering application. Adv. Drug Deliv. Rev. 2007, 59, 207–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dionísio, M.; Grenha, A. Locust bean gum: Exploring its potential for biopharmaceutical applications. J. Pharm. Bioallied Sci. 2012, 4, 75–85. [Google Scholar]
- Kawamura, Y. Carob Bean Gum: Chemical and technical Assessment; Joint FAO/WHO Expert Committee on Food Additives: Geneva, Switzerland, 2008. [Google Scholar]
- Mathur, V.; Mathur, N. Fenugreek and other less known legume galactomannan-polysaccharides: Scope for developments. J. Sci. Ind. Res. 2005, 64, 475–481. [Google Scholar]
- Arpagaus, C.; Schafroth, N.; Meuri, M. Laboratory scale spray drying of inhalable drugs: A review. Buchi: Flawil, Switzerland.
- Alves, M.M.; Antonov, Y.A.; Gonçalves, M.P. The effect of structural features of gelatin on its thermodynamic compatibility with locust bean gum in aqueous media. Food Hydrocoll. 1999, 13, 157–166. [Google Scholar] [CrossRef]
- Farahnaky, A.; Darabzadeh, N.; Majzoobi, M.; Mesbahi, G. Physicochemical properties of crude and purified locust bean gums extracted from Iranian carob seeds. J. Agric. Sci. Technol. 2014, 16, 125–136. [Google Scholar]
- Surendrakumar, K.; Martyn, G.P.; Hodgers, E.C.M.; Jansen, M.; Blair, J.A. Sustained release of insulin from sodium hyaluronate based dry powder formulations after pulmonary delivery to beagle dogs. J. Control. Release 2003, 91, 385–394. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Yeh, M.-K.; Chiang, C.-H. Formulation factors in preparing BTM-chitosan microspheres by spray drying method. Int. J. Pharm. 2002, 242, 239–242. [Google Scholar] [CrossRef]
- Grenha, A.; Seijo, B.; Remuñán-López, C. Microencapsulated chitosan nanoparticles for lung protein delivery. Eur. J. Pharm. Sci. 2005, 25, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Al-Qadi, S.; Grenha, A.; Remuñán-López, C. Microspheres loaded with polysaccharide nanoparticles for pulmonary delivery: Preparation, structure and surface analysis. Carbohydr. Polym. 2011, 86, 25–34. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, H.-L.; Hu, Y.-M.; Wan, S.; Su, Z.-Q. Preparation of chitosan and water-soluble chitosan microspheres via spray-drying method to lower blood lipids in rats fed with high-fat diets. Int. J. Mol. Sci. 2014, 14, 4174–4184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ameri, M.; Maa, Y.-F. Spray drying of biopharmaceuticals: Stability and process considerations. Dry. Technol. 2006, 24, 763–768. [Google Scholar] [CrossRef]
- Yang, M.Y.; Chan, J.G.Y.; Chan, H.-K. Pulmonary drug delivery by powder aerosols. J. Control. Release 2014, 193, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Maury, M.; Murphy, K.; Kumar, S.; Shi, L.; Lee, G. Effects of process variables on the powder yield of spray-dried trehalose on a laboratory spray-dryer. Eur. J. Pharm. Biopharm. 2005, 59, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.; Kellaway, I. Pulmonary drug delivery. In Drug Delivery and Targeting; Hillery, A., Lloyd, A., Swarbrick, J., Eds.; Taylor & Francis: New York, NY, USA, 2001; pp. 269–300. [Google Scholar]
- Rassu, G.; Soddu, E.; Cossu, M.; Brundu, A.; Cerri, G.; Marchetti, N.; Ferraro, L.; Regan, R.F.; Giunchedi, P.; Gavini, E.; et al. Solid microparticles based on chitosan or methyl-β-cyclodextrin: A first formulative approach to increase the nose-to-brain transport of deferoxamine mesylate. J. Control. Release 2015, 201, 68–77. [Google Scholar] [CrossRef]
- Palazzo, F.; Giovagnoli, S.; Schoubben, A.; Blasi, P.; Rossi, C.; Ricci, M. Development of a spray-drying method for the formulation of respirable microparticles containing ofloxacin–palladium complex. Int. J. Pharm. 2013, 440, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Pant, G.; Mitra, K.; Madan, J.; Chourasia, M.K.; Misra, A. Inhalable particles containing rapamycin for induction of autophagy in macrophages infected with Mycobacterium tuberculosis. Mol. Pharm. 2014, 11, 1201–1207. [Google Scholar] [CrossRef]
- Sollohub, K.; Cal, K. Spray drying technique: II. Current applications in pharmaceutical technology. J. Pharm. Sci. 2010, 99, 587–597. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Biggs, D.L.; Manning, M.C.; Randolph, T.W.; Christians, U.; Hybertson, B.M.; Ng, K.-Y. Microparticle-based lung delivery of INH decreases INH metabolism and targets alveolar macrophages. J. Control. Release 2005, 107, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Kundawala, A.; Patel, V.; Patel, H.; Choudhary, D. Isoniazid loaded chitosan microspheres for pulmonary delivery: Preparation and characterization. Pharm. Sin. 2011, 2, 88–97. [Google Scholar]
- Chan, J.G.Y.; Chan, H.-K.; Prestidge, C.A.; Denman, J.A.; Young, P.M.; Traini, D. A novel dry powder inhalable formulation incorporating three first-line anti-tubercular antibiotics. Eur. J. Pharm. Biopharm. 2013, 83, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Shanmuga Priya, A.; Sivakamavalli, J.; Vaseeharan, B.; Stalin, T. Improvement on dissolution rate of inclusion complex of Rifabutin drug with β-cyclodextrin. Int. J. Biol. Macromol. 2013, 62, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Giri, T.; Pure, S.; Tripathi, D. Synthesis of graft copolymers of acrylamide for locust bean gum using microwave energy: Swelling behavior, flocculation characteristics and acute toxicity study. Polímeros 2015, 25, 168–174. [Google Scholar] [CrossRef]
- Angadi, S.C.; Manjeshwar, L.S.; Aminabhavi, T.M. Interpenetrating polymer network blend microspheres of chitosan and hydroxyethyl cellulose for controlled release of isoniazid. Int. J. Biol. Macromol. 2010, 47, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Ógáin, O.N.; Li, J.; Tajber, L.; Corrigan, O.I.; Healy, A.M. Particle engineering of materials for oral inhalation by dry powder inhalers. I—Particles of sugar excipients (trehalose and raffinose) for protein delivery. Int. J. Pharm. 2011, 405, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Wuelfing, P.; Kosuda, K.; Templeton, A.C.; Harman, A.; Mowery, M.D.; Reed, R.A. Polysorbate 80 UV/vis spectral and chromatographic characteristics—Defining boundary conditions for use of the surfactant in dissolution analysis. J. Pharm. Biomed. Anal. 2006, 41, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, D.; Patel, A.; Shah, S. Influence of selected natural polymers on in vitro release of colon targeted mebeverine HCl matrix tablet. Int. J. Drug Dev. Res. 2012, 4, 315–321. [Google Scholar]
- De Souza Carvalho, C.; Daum, N.; Lehr, C.-M. Carrier interactions with the biological barriers of the lung: Advanced in vitro models and challenges for pulmonary drug delivery. Adv. Drug Deliv. Rev. 2014, 75, 129–140. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization. Biological evaluation of medical devices Part 5: Tests for in vitro cytotoxicity. In ISO 10993-5; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Gaspar, R.; Duncan, R. Polymeric carriers: Preclinical safety and the regulatory implications for design and development of polymer therapeutics. Adv. Drug Deliv. Rev. 2009, 61, 1220–1231. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.; Dalwadi, S.; Aboti, P.; Patel, L. Inhaled microparticles of antitubercular antibiotic for in vitro and in vivo alveolar macrophage targeting and activation of phagocytosis. J. Antibiot. 2014, 67, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Bhatnagar, P.; Mishra, S.; Kumar, P.; Shukla, Y.; Gupta, K.C. PLGA-encapsulated tea polyphenols enhance the chemotherapeutic efficacy of cisplatin against human cancer cells and mice bearing Ehrlich ascites carcinoma. Int. J. Nanomed. 2015, 10, 6789–6809. [Google Scholar] [CrossRef] [PubMed]
- Lankoff, A.; Sandberg, W.J.; Wegierek-Ciuk, A.; Lisowska, H.; Refsnes, M.; Sartowska, B.; Schwarze, P.E.; Meczynska-Wielgosz, S.; Wojewodzka, M.; Kruszewski, M. The effect of agglomeration state of silver and titanium dioxide nanoparticles on cellular response of HepG2, A549 and THP-1 cells. Toxicol. Lett. 2012, 208, 197–213. [Google Scholar] [CrossRef] [PubMed]
- Lanone, S.; Rogerieux, F.; Geys, J.; Dupont, A.; Maillot-Marechal, E.; Boczkowski, J.; Lacroix, G.; Hoet, P. Comparative toxicity of 24 manufactured nanoparticles in human alveolar epithelial and macrophage cell lines. Part. Fibre Toxicol. 2009, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barluenga, J.; Aznar, F.; García, A.-B.; Cabal, M.-P.; Palacios, J.J.; Menéndez, M.-A. New rifabutin analogs: Synthesis and biological activity against Mycobacterium tuberculosis. Bioorg. Med. Chem. Lett. 2006, 16, 5717–5722. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.; Arêde, M.; Nunes, C.; Caio, J.; Moiteiro, C.; Lúcio, M.; Reis, S. Differential interactions of rifabutin with human and bacterial membranes: Implication for its therapeutic and toxic effects. J. Med. Chem. 2013, 56, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Widdicombe, J. Volume of airway surface liquid in health and disease. Am. J. Resp. Crit. Care Med. 2002, 165, 1566. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, M.; Nunes, C.; Caio, J.M.; Moiteiro, C.; Lúcio, M.; Brezesinski, G.; Reis, S. The influence of rifabutin on human and bacterial membrane models: Implications for its mechanism of action. J. Phys. Chem. B 2013, 117, 6187–6193. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Sande, M.; Teijeiro-Osorio, D.; Remuñán-López, C.; Alonso, M.J. Glucomannan, a promising polysaccharide for biopharmaceutical purposes. Eur. J. Pharm. Biopharm. 2009, 72, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Alkhayat, A.H.; Kraemer, S.A.; Leipprandt, J.R.; Macek, M.; Kleijer, W.J.; Friderici, K.H. Human β-mannosidase cDNA characterization and first identification of a mutation associated with human β-mannosidosis. Hum. Mol. Genet. 1998, 7, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Geiser, M. Update on macrophage clearance of inhaled micro- and nanoparticles. J. Aerosol Med. Pulm. Drug Deliv. 2010, 23, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Costa-Lima, S.; Carneiro, T.; Cordeiro-da-Silva, A.; Souto, E.B.; Ferreira, D.C. Solid lipid nanoparticles as intracellular drug transporters: An investigation of the uptake mechanism and pathway. Int. J. Pharm. 2012, 430, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Gupta, N.; Ahsan, F. Particle engineering to enhance or lessen particle uptake by alveolar macrophages and to influence the therapeutic outcome. Eur. J. Pharm. Biopharm. 2015, 89, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, S.; Grenha, A. Activation of macrophages: Establishing a role for polysaccharides in drug delivery strategies envisaging antibacterial therapy. Curr. Pharm. Des. 2015, 21, 4869–4887. [Google Scholar] [CrossRef] [PubMed]
- Pollard, M.; Kelly, R.; Fischer, P.; Windhab, E.; Eder, B.; Amadò, R. Investigation of molecular weight distribution of LBG galactomannan for flours prepared from individual seeds, mixtures, and commercial samples. Food Hydrocoll. 2008, 22, 1596–1606. [Google Scholar] [CrossRef]
- Bosquillon, C.; Préat, V.; Vanbever, R. Pulmonary delivery of growth hormone using dry powders and visualization of its local fate in rats. J. Control. Release 2004, 96, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, P.S.; Saha, R.N. Controlled release hydrophilic matrix tablet formulations of isoniazid: Design and in vitro studies. AAPS PharmSciTech 2008, 9, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Guideline on Quality of Oral Modified Release Products; EMA: London, UK, 2014; Volume EMA/CHMP/QWP/428693/2013, pp. 1–16. [Google Scholar]
- Sample Availability: Not available.











| Formulation | LBG/Drug (w/w) | Production Yield (%) | Diameter (μm) | ||
|---|---|---|---|---|---|
| Feret’s | Aerodynamic, λ = 1 | Aerodynamic, λ = 2 | |||
| Unloaded LBG | 10:0 | 70.1 ± 4.1 | 1.35 ± 0.73 | 1.59 ± 0.06 | 1.12 ± 0.04 |
| LBG:INH | 10:1 | 66.0 ± 5.8 | 1.50 ± 0.80 | 1.83 ± 0.21 | 1.30 ± 0.16 |
| LBG:RFB | 10:0.2 | 61.5 ± 0.7 | 1.26 ± 0.63 | 1.54 ± 0.21 | 1.09 ± 0.16 |
| 10:0.5 | 67.0 ± 2.8 | 1.10 ± 0.56 | 1.27 ± 0.01 | 0.89 ± 0.01 | |
| 10:1 | 70.1 ± 4.8 | 1.50 ± 0.86 | 1.78 ± 0.03 | 1.26 ± 0.03 | |
| Formulation | LBG/Drug (w/w) | Density (g/cm3) | ||
|---|---|---|---|---|
| Real | Bulk | Tap | ||
| Unloaded LBG | 10:0 | 1.39 ± 0.01 | 0.24 ± 0.06 | 0.37 ± 0.08 |
| LBG:INH | 10:1 | 1.41 ±0.02 | 0.24 ± 0.01 | 0.36 ± 0.00 |
| LBG:RFB | 10:0.2 | 1.41 ± 0.03 | 0.20 ± 0.01 | 0.32 ± 0.05 |
| 10:0.5 | 1.33 ± 0.03 | 0.15 ± 0.04 | 0.25 ± 0.07 | |
| 10:1 | 1.39 ± 0.02 | 0.14 ± 0.02 | 0.25 ± 0.02 | |
| Formulation | LBG/Drug (w/w) | Association Efficiency (%) | Loading Capacity (%) |
|---|---|---|---|
| LBG:INH | 10:1 | 88.8 ± 1.5 | 8.8 ± 0.1 |
| LBG:RFB | 10:0.2 | 92.4 ± 6.0 | 1.8 ± 0.1 |
| 10:0.5 | 86.3 ± 3.0 | 4.1 ± 0.1 | |
| 10:1 | 102.8 ± 3.8 | 10.3 ± 0.4 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, A.D.; Cavaco, J.S.; Guerreiro, F.; Lourenço, J.P.; Rosa da Costa, A.M.; Grenha, A. Inhalable Antitubercular Therapy Mediated by Locust Bean Gum Microparticles. Molecules 2016, 21, 702. https://doi.org/10.3390/molecules21060702
Alves AD, Cavaco JS, Guerreiro F, Lourenço JP, Rosa da Costa AM, Grenha A. Inhalable Antitubercular Therapy Mediated by Locust Bean Gum Microparticles. Molecules. 2016; 21(6):702. https://doi.org/10.3390/molecules21060702
Chicago/Turabian StyleAlves, Ana D., Joana S. Cavaco, Filipa Guerreiro, João P. Lourenço, Ana M. Rosa da Costa, and Ana Grenha. 2016. "Inhalable Antitubercular Therapy Mediated by Locust Bean Gum Microparticles" Molecules 21, no. 6: 702. https://doi.org/10.3390/molecules21060702
APA StyleAlves, A. D., Cavaco, J. S., Guerreiro, F., Lourenço, J. P., Rosa da Costa, A. M., & Grenha, A. (2016). Inhalable Antitubercular Therapy Mediated by Locust Bean Gum Microparticles. Molecules, 21(6), 702. https://doi.org/10.3390/molecules21060702