The Deformation of Polydimethylsiloxane (PDMS) Microfluidic Channels Filled with Embedded Circular Obstacles under Certain Circumstances
Abstract
:1. Introduction
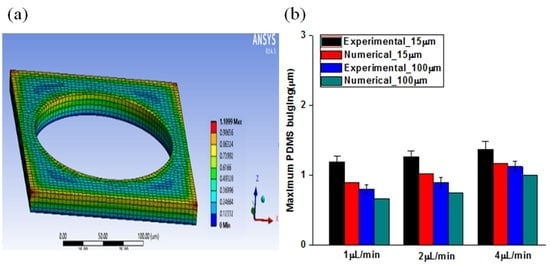
2. Results and Discussion
3. Materials and Methods
3.1. Procedures
3.2. Data Analysis
3.3. Finite Element Model
4. Conclusions
- (1)
- The geometry of the microfluidic channels: 15 µm microfluidic channels generated an unexpected discrepancy in the flow velocity between the theoretical and experimental results compared to the 100 µm microfluidic channels. In addition, the diameter of the embedded obstacles (i.e., FC2 and FC4) influenced the flow behaviors. For example, large embedded obstacles (i.e., FC2) caused a substantial discrepancy from the predicted values.
- (2)
- Operational flow conditions: The flow operated in steady-state induced much less PDMS bulging and followed the classical theory compared to quasi-steady state flow.
- (3)
- Applied flow rates: sizeable PDMS bulging was observed at high flow rates, and the discrepancy from the predicted values became severe as the flow rates increased.
- (4)
- PDMS mixing ratios: The PDMS mixing ratios were found to be a controlling factor in changing the material property. Increasing stiffness by increasing curing agent (B) led to the following of the theoretical values of the flow velocity and pressure drop, whereas PDMS microfluidic channel by decreasing curing agent began to generate a noticeable discrepancy.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Melin, J.; Quake, S.R. Microfluidic large-scale integration: The evolution of design rules for biological automation. Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Meng, T.; Li, W.; Qin, J.; Tong, Z.; Zhang, Q.; Li, X. UV-driven microvalve based on a micro-nanoTiO2/SiO2 composite surface for microscale flow control. Nanotechnology 2014, 25, 12530. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E.K.; Fulton, A.L.D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cui, J.; Li, W.Z.; Zhang, N. Effect of surface microstructure on microchannel heat transfer performance. J. Heat Transf. 2011, 133, 124501. [Google Scholar] [CrossRef]
- Peng, S.W.; Revellin, R.; Groeneveld, D.C.; Vasic, A.Z.; Shang, D.; Cheng, S.C. Effects of flow obstacles on film boiling heat transfer. Nucl. Eng. Des. 2003, 222, 89–95. [Google Scholar] [CrossRef]
- Peles, Y.; Kosar, A.; Mishra, C.; Kuo, C.; Schneider, B. Force convective heat transfer across a pin fin micro heat sink. Int. J. Heat Mass Transf. 2005, 48, 3615–3627. [Google Scholar] [CrossRef]
- Roth, R.; Lenk, G.; Cobry, K.; Woias, P. Heat transfer in freestanding microchannels with in-line and staggered pin fin structures with clearance. Int. J. Heat Mass Transf. 2013, 67, 1–15. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Peles, Y. Flow boiling heat transfer on micro pin fins entrenched in a microchannel. J. Heat Transf. 2010, 132, 041007. [Google Scholar] [CrossRef]
- Bhagat, A.A.S.; Peterson, E.T.K.; Papautsky, I. A passive planar micromixer with obstructions for mixing at low Reynolds numbers. J. Micromech. Microeng. 2007, 17, 1017–1024. [Google Scholar] [CrossRef]
- Bhagat, A.A.S.; Papautsky, I. Enhancing particle dispersion in a passive planar micromixer using rectangular obstacles. J. Microeng. Micromech. 2008, 18, 085005. [Google Scholar] [CrossRef]
- Jain, M.; Nandakumar, K. Novel index for micromixing characterization and comparative analysis. Biomicrofluidics 2010, 4, 031101. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wu, X.; Lin, F. Recent developments in microfluidics-based chemotaxis studies. Lab Chip 2013, 13, 2484–2899. [Google Scholar] [CrossRef] [PubMed]
- Long, T.; Ford, R.M. Enhanced transverse migration of bacteria by chemotaxis in a porous T-sensor. Environ. Sci. Technol. 2009, 43, 1546–1552. [Google Scholar] [CrossRef] [PubMed]
- Kao, P.; Ren, T.; Yang, R. An investigation into fixed-bed microreactors using lattice Boltzmann method simulations. Int. J. Heat Mass Transf. 2007, 50, 4243–4255. [Google Scholar] [CrossRef]
- Marty, A.; Roques, C.; Causserand, C.; Bacchin, P. Formation of bacterial streamers during filtration in microfluidic systems. Biofouling 2012, 28, 551–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Jiang, P.; Xu, R. Experimental Investigation of Fluid Flow and Internal convection heat transfer in mini/micro porous media. In Proceedings of the ASME 2009 Second International Conference on Micro/Nanoscale Heat and Mass Transfer, Shanghai, China, 18–21 December 2009.
- Li, B.; Zhou, W.N.; Yan, Y.Y.; Tian, C. Evaluation of electro-osmotic pumping effect on microporous media flow. Appl. Therm. Eng. 2013, 60, 449–455. [Google Scholar] [CrossRef]
- Holdich, R.; Kosvintsev, S.; Cumming, I.; Zhdanov, S. Pore design and engineering for filters and membranes. Phil. Trans. R. Soc. A 2006, 364, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Tamayol, A.; Khosla, A.; Gray, B.L.; Bahrami, M. Creeping flow through ordered arrays of micro-cylinders embeddedin a rectangular minichannel. Int. J. Heat Mass Transf. 2012, 55, 3900–3908. [Google Scholar] [CrossRef]
- Chu, J.C.; Teng, J.T.; Greif, R. Experimental and numerical study on the flow characteristics in curved rectangular microchannels. Appl. Therm. Eng. 2010, 30, 1558–1566. [Google Scholar] [CrossRef]
- Koo, J.; Kleinstreuer, C. Liquid flow in microchannels: Experimental observations and computational analyses of microfluidics effects. J. Micromech. Microeng. 2003, 13, 568–579. [Google Scholar] [CrossRef]
- Steinke, M.E.; Kandlikar, S.G. Review of single-phase heat transfer enhancement techniques for application in microchannels, minichannels and microdevices. Int. J. Heat Technol. 2004, 22, 3–11. [Google Scholar]
- Steinke, M.E.; Kandlikar, S.G. Single-phase liquid friction factors in microchannels. Int. J. Therm. Sci. 2006, 45, 1073–1083. [Google Scholar] [CrossRef]
- Kang, C.; Roh, C.; Overfelt, R.A. Pressure-driven deformation with soft polydimethylsiloxane (PDMS) by a regular syringe pump; challenge to the classical fluid dynamics by comparison of experimental and theoretical results. RSC Adv. 2014, 4, 3102–3112. [Google Scholar] [CrossRef]
- Aniskin, V.M.; Adamenko, K.V.; Maslov, A.A. Experimental determination of the friction factor coefficient in microchannels. J. Appl. Mech. Tech. Phys. 2011, 52, 18–23. [Google Scholar] [CrossRef]
- Mirmanto, D.B.R. Developing flow pressure drop and friction factor. J. Mech. Eng. Autom. 2013, 3, 641–649. [Google Scholar]
- Kohl, M.J.; Abdel-Khalik, S.I.; Jeter, S.M.; Sadowski, D.L. An experimental investigation of microchannel flow with internal pressure measurements. Int. J. Heat Mass Transf. 2005, 48, 1518–1533. [Google Scholar] [CrossRef]
- Puccettik, G.; Pulvirenti, B.; Morini, G.L. Use of the μPIV technique for an indirect determination of the microchannel cross-section passage geometry. J. Phys.: Conf. Ser. 2014, 501, 012027. [Google Scholar]
- Akbari, M.; Sinton, D.; Bahrami, M. Pressure drop in rectangular microchannelsas compared with theory based on arbitrary cross section. J. Fluid Eng. 2009, 131, 041202. [Google Scholar] [CrossRef]
- Schneider, F.; Draheim, J.; Kamberger, R.; Wallrabe, U. Process and material properties of polydimethylsiloxane (PDMS) for optical MEMS. Sens. Actuators A: Phys. 2009, 151, 95–99. [Google Scholar] [CrossRef]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Shueller, O.J.; Whitesides, G.M. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Bhagat, A.A.S.; Jothimuthu, P.; Papautsky, I. Photodefinable polydimethylsiloxane (PDMS) for rapid lab-on- a-chip prototyping. Lab Chip 2007, 7, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Gervais, T.; El-Ali, J.; Gunther, A.; Jensen, K.F. Flow-induced deformation of shallow microfluidic channels. Lab Chip 2006, 6, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Hardy, B.S.; Uechi, K.; Zhen, J.; Kavehpour, H.P. The deformation of flexible PDMS microchannels under a pressure driven flow. Lab Chip 2009, 7, 935–938. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.; Toda-Peters, K.; Shen, A. In situ pressure measurement within deformable rectangular polydimethylsiloxane microfluidic devices. Biomicrofluidics 2012, 6, 026501. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Huang, Y.; Choi, K.; Hidrovo, C.H. The improved resistance of PDMS to pressure-induced deformation and chemical solvent swelling for microfluidic devices. Microelecronic Eng. 2014, 124, 66–75. [Google Scholar] [CrossRef]
- Holden, M.A.; Kumar, S.; Beskok, A.; Cremer, P.S. Microfluidic diffusion diluter: Bulging of PDMS microchannels under the pressure driven flow. J. Micromech. Microeng. 2003, 13, 412–418. [Google Scholar] [CrossRef]
- Ross, D.; Gaitan, M.; Locascio, L.E. Temperature measurement in microfluidic systems using a temperature-dependent fluorescent dye. Anal. Chem. 2001, 73, 4117–4123. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Liu, Q.; Li, Y. Capillary filling inside patterned-surface microchannels. Chem. Eng. Technol. 2006, 29, 716–723. [Google Scholar] [CrossRef]
- Tamayol, A.; Yeom, J.; Akbari, M.; Bahrami, M. Low Reynolds number flows across ordered arrays of micro-cylinders embedded in a rectangular micro/minichannel. Int. J. Heat Mass Transf. 2013, 58, 420–426. [Google Scholar] [CrossRef]
- Yazdchi, K.; Srivastava, S.; Luding, S. Microstructural effects on the permeability of periodic fibrous porous media. Int. J. Multi. Flow 2011, 37, 956–966. [Google Scholar] [CrossRef]
- White, F.M. Viscous Fluid Flow; McGraq-Hill: New York, NY, USA, 1994; pp. 1–120. [Google Scholar]
- Dadkhah, F.; Zecher, J. ANSYS Workbench Software; SDS Publications: Mission, KS, USA, 2009; pp. 1–160. [Google Scholar]
- Choi, S.; Park, J. Tuneablehydrophoretic separation using elastic deformation of poly (dimethylsiloxane). Lab Chip 2009, 9, 1962–1965. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not avalable.







| Obstacle and Depth | Diameter of Obstacle (mm) | Overall Microchannel Length (mm) | Mean Porosity (%) | Mean Hydraulic Diameter (µm) |
|---|---|---|---|---|
| FC2_15 µm | 0.172 | 10.1 | 54 | 23.7 |
| FC4_15 µm | 0.132 | 9.14 | 70 | 25.2 |
| FC2_100 µm | 0.172 | 10.1 | 54 | 71.9 |
| FC4_100 µm | 0.132 | 9.14 | 70 | 88.5 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roh, C.; Lee, J.; Kang, C. The Deformation of Polydimethylsiloxane (PDMS) Microfluidic Channels Filled with Embedded Circular Obstacles under Certain Circumstances. Molecules 2016, 21, 798. https://doi.org/10.3390/molecules21060798
Roh C, Lee J, Kang C. The Deformation of Polydimethylsiloxane (PDMS) Microfluidic Channels Filled with Embedded Circular Obstacles under Certain Circumstances. Molecules. 2016; 21(6):798. https://doi.org/10.3390/molecules21060798
Chicago/Turabian StyleRoh, Changhyun, Jaewoong Lee, and Chankyu Kang. 2016. "The Deformation of Polydimethylsiloxane (PDMS) Microfluidic Channels Filled with Embedded Circular Obstacles under Certain Circumstances" Molecules 21, no. 6: 798. https://doi.org/10.3390/molecules21060798
APA StyleRoh, C., Lee, J., & Kang, C. (2016). The Deformation of Polydimethylsiloxane (PDMS) Microfluidic Channels Filled with Embedded Circular Obstacles under Certain Circumstances. Molecules, 21(6), 798. https://doi.org/10.3390/molecules21060798