An Ingenol Derived from Euphorbia kansui Induces Hepatocyte Cytotoxicity by Triggering G0/G1 Cell Cycle Arrest and Regulating the Mitochondrial Apoptosis Pathway in Vitro
Abstract
:1. Introduction
2. Results
2.1. Effects of 3EZ, 20Ac-Ingenol on L-O2 Cell Viability and Cell Morphology
2.2. Effects of 3EZ, 20Ac-Ingenol on L-O2 Cell Cycle and Apoptosis
2.3. Effects of 3EZ, 20Ac-Ingenol on the Generation of Reactive Oxygen Species (ROS)
2.4. Effects of 3EZ, 20Ac-Ingenol on Mitochondrial Function
2.5. Effects of 3EZ, 20Ac-Ingenol on Caspases Activation
2.6. Effects of 3EZ, 20Ac-Ingenol on Mitochondrial Apoptotic Proteins
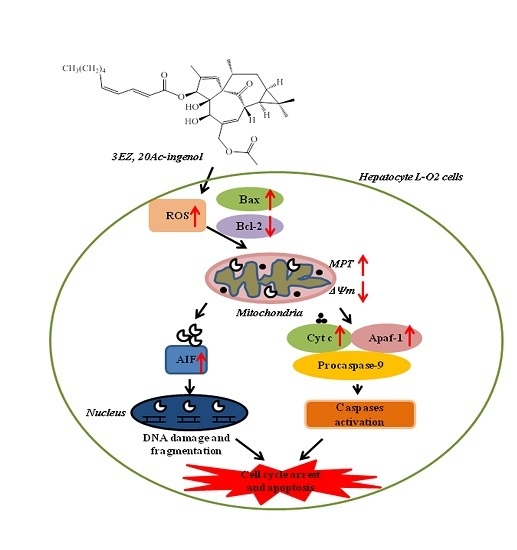
3. Discussions
4. Experimental Section
4.1. Plant Materials
4.2. Preparation of Sample Solutions
4.3. Chemical and Reagents
4.4. Cell Line and Cell Culture
4.5. Cell Viability Analysis
4.6. Cell Morphological Detection
4.7. Cell Cycle Analysis
4.8. Cell Apoptosis Analysis
4.9. Intracellular Reactive Oxygen Species (ROS) Detection
4.10. High Content Screening (HCS) Analysis of Mitochondrial Function
4.11. Elisa Assay
4.12. Western Blot Analysis
4.13. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, F.F.; Yang, Y.Y.; Zhang, L.; Cao, Y.D.; Yao, W.F.; Tang, Y.P.; Ding, A.W. A natural triterpene derivative from Euphorbia kansui inhibits cell proliferation and induces apoptosis against rat intestinal epithelioid cell line in vitro. Int. J. Mol. Sci. 2015, 16, 18956–18975. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Wang, J.S.; Wei, D.D.; Wang, X.B.; Luo, J.; Yang, M.H.; Kong, L.Y. Bioactivity-guided isolation of antiproliferative diterpenoids from Euphorbia kansui. Phytother. Res. 2012, 26, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.F.; Cui, Z.; Zhu, Q. Cytotoxicity and antiviral activity of the compounds from Euphorbia kansui. Planta Med. 1998, 64, 754–756. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhong, J.M.; Ye, S.Q.; Ni, Z.Y.; Miao, X.Q.; Mo, Y.K.; Li, Z.L. Screening of Epstein-Barrvirus early antigen expression inducers from Chinese medicinal herbs and plants. Biomed. Environ. Sci. 1994, 7, 50–55. [Google Scholar] [PubMed]
- Nunomura, S.; Kitanaka, S.; Ra, C. 3-O-(2,3-dimethylbutanoyl)-13-O-decan-oylingenol from Euphorbia kansui suppresses IgE-mediated mast cell activation. Biol. Pharm. Bull. 2006, 29, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Khiev, P.; Kim, J.W.; Sung, S.J.; Song, H.H.; Choung, D.H.; Chin, Y.W.; Lee, H.; Oh, S.R. Ingenane-type Diterpenes with a Modulatory Effect on IFN-γ Production from the Roots of Euphorbia kansui. Arch. Pharm. Res. 2012, 35, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhou, L.Y.; He, H.P.; Leng, Y.; Yang, Z.; Hao, X.J. Inhibition of 11b-HSD1 by tetracyclic triterpenoids from Euphorbia kansui. Molecules 2012, 17, 11826–11838. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.W.; Ding, J.J.; Yang, Y.X.; Wu, F.H.; Song, F.Y. Systems biochemical responses of rats to kansui and vinegar-processed kansui exposure by integrated metabonomics. J. Ethnopharmacol. 2014, 153, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.W.; Ding, J.J.; Wu, F.H.; Chen, L.; Yang, Y.X.; Song, F.Y. 1H-NMR-based metabonomics study of the urinary biochemical changes in kansui treated rat. J. Ethnopharmacol. 2012, 141, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; He, H.P.; Fang, X. Kansuinone, a novel euphane-type triterpene from Euphorbia kansui. Tetrahedron Lett. 2010, 51, 6286–6289. [Google Scholar] [CrossRef]
- Chang, J.S.; Lee, S.W.; Park, M.H.; Kim, M.S.; Hudson, B.I.; Park, S.J.; Lee, W.S. Kansuinine A and Kansuinine B from Euphorbia kansui L. inhibit IL-6-induced Stat3 activation. Planta Med. 2010, 76, 1544–1549. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Ip, F.C.; Ip, N.Y.; Zhu, H.X.; Min, Z.D. Activity of macrocyclic jatrophane diterpenes from Euphorbia kansui in a TrkA fibroblast survival assay. J. Nat. Prod. 2004, 67, 1548–1551. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Wang, N.L.; Yao, X.S.; Miyata, S.; Kitanaka, S. Euphane and tirucallane triterpenes from the roots of Euphorbia kansui and their in vitro effects on the cell division of Xenopus. J. Nat. Prod. 2003, 66, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Dang, Q.L.; Choi, Y.H.; Choi, G.J.; Jang, K.S.; Park, M.S.; Park, N.J.; Lim, C.H.; Kim, H.; Ngoc, L.H.; Kim, J.C. Pesticidal activity of ingenane diterpenes isolated from Euphorbia kansui against Nilaparvata lugens and Tetranychus urticae. J. Asia Pac. Entomol. 2010, 13, 51–54. [Google Scholar] [CrossRef]
- Wu, T.S.; Lin, Y.M.; Haruna, M.; Pan, D.J.; Shingu, T.; Chen, T.P.; Hsu, H.Y.; Nakano, T.; Lee, K.H. Antitumor agent, 119 kansuiphorins A and B, two novel antileukemic diterpene ester from Euphorbia kansui. J. Nat. Prod. 1991, 54, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.J.; Zhang, L.; Guo, J.M.; Cao, Y.D.; Shang, E.X.; Tang, Y.P.; Ding, A.W.; Duan, J.A. Processing of kansui roots stir-baked with vinegar reduces kansui-induced hepatocyte cytotoxicity by decreasing the contents of toxic terpenoids and regulating the cell apoptosis pathway. Molecules 2014, 19, 7237–7254. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gao, L.; Li, Z.J.; Yan, X.J.; Yang, Y.J.; Tang, Y.P.; Cao, Y.D.; Ding, A.W. Bio-Guided isolation of the cytotoxic terpenoids from the roots of Euphorbia kansui against human normal cell lines L-O2 and GES-1. Int. J. Mol. Sci. 2012, 13, 11247–11259. [Google Scholar] [CrossRef] [PubMed]
- Ockner, R.K. Apoptosis and liver diseases: Recent concepts of mechanism and significance. J. Gastroenterol. Hepatol. 2001, 16, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Matthews, G.M.; Newbold, A.; Johnstone, R.W. Intrinsic and extrinsic apoptotic pathway signaling as determinants of histone deacetylase inhibitor antitumor activity. Adv. Cancer Res. 2012, 116, 165–197. [Google Scholar] [PubMed]
- Pessayre, D.; Mansouri, A.; Berson, A.; Fromentry, B. Mitochondrial involvement in drug-induced liver injury. Handb. Exp. Pharmacol. 2010, 196, 311–365. [Google Scholar] [PubMed]
- Forbes-Hernandez, T.Y.; Giampieri, F.; Gasparrini, M.; Mazzoni, L.; Quiles, J.L.; Alvarez-Suarez, J.M.; Battino, M. The effects of bioactive compounds from plant foods on mitochondrial function: A focus on apoptotic mechanisms. Food Chem. Toxicol. 2014, 68, 154–182. [Google Scholar] [CrossRef] [PubMed]
- Gross, A. Bcl-2 family proteins as regulators of mitochondria metabolism. Biochim. Biophys. Acta 2016, 1857, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Simon, H.U.; Haj-Yehia, A.; Levi-Schaffer, F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis 2000, 5, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Thannickal, V.J.; Fanburg, B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung C 2000, 279, L1005–L1028. [Google Scholar]
- Joza, N.; Susin, S.A.; Daugas, E.; Stanford, W.L.; Cho, S.K.; Li, C.Y.; Sasaki, T.; Elia, A.J.; Cheng, H.Y.; Ravagnan, L.; et al. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature 2001, 410, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Dumont, C.; Durrbach, A.; Bidere, N.; Rouleau, M.; Kroemer, G.; Bernard, G.; Susin, S.A.; Senik, A. Caspase-independent commitment to apoptosis in activated blood T lymphocytes: Reversibility at low apoptotic insult. Blood 2000, 96, 1030–1038. [Google Scholar] [PubMed]
- Makani, S.; Gollapudi, S.; Yel, L.; Chiplunfar, S.; Gupta, S. Biochemical and molecular basis of thimerosal-induced apoptosis in T cells: A major role of mitochondrial pathway. Genes Immun. 2002, 3, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Pardo, J.; Perez-Galan, P.; Gamen, S.; Marzo, I.; Monleon, I.; Kaspar, A.A. A role of the mitochondrial apoptosis-inducing factor in granulysin-induced apoptosis. J. Immunol. 2001, 167, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.; Cory, S. Apoptosomes: Engines for caspase activation. Curr. Opin. Cell Biol. 2002, 14, 715–720. [Google Scholar] [CrossRef]
- Ando, K.; Hiroishi, K.; Kaneko, T. Perforin, Fas/Fas ligand, and TNF-alpha pathways as specific and bystander killing mechanisms of hepatitis C virus-specific human CTL. J. Immunol. 1997, 158, 5283–5291. [Google Scholar] [PubMed]
- Hiramatsu, N.; Hayashi, N.; Katayama, K.; Mochizuki, K.; Kawanishi, Y.; Kasahara, A. Immunohistochemical detection of Fas antigen in liver tissue of patients with chronic hepatitis C. Hepatology 1994, 19, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.; Gores, G.J. Apoptosis and hepatobiliary disease. Hepatology 1995, 21, 1725–1741. [Google Scholar] [PubMed]
- Bernd, M.; Rainer, O. Mitochondrial Regulation of Apoptosis. News Physiol. Sci. 2003, 18, 89–94. [Google Scholar]
- Zamzani, N.; Kroemer, G. The mitochondrion in apoptosis: How Pandora′s box opens. Nat. Rev. 2001, 2, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.J.; Wang, X.D. Cytochrome c-mediated apoptosis. Annu. Rev. Biochem. 2004, 73, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Susin, S.A.; Lorenzo, H.K.; Zamzami, N.; Marzo, I.; Snow, B.E.; Brothers, G.M.; Mangion, J.; Jacotot, E.; Costantini, P.; Loeffler, M.; et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999, 397, 441–446. [Google Scholar] [PubMed]
- Daugas, E.; Susin, S.A.; Zamzami, N.; Frrri, K.F.; Irinopoulou, T.; Larochette, N.; Prevost, M.C.; Leber, B.; Andrews, D.; Penninger, J.; et al. Mitochondria-nuclear translocation of AIF in apoptosis and necrosis. FASEB J. 2000, 14, 729–739. [Google Scholar] [PubMed]
- Hellstrom-Lindberg, E.; Schmidt-Mende, J.; Forsblom, A.M.; Christensson, B.; Fadeel, B.; Zhivotovsky, B. Apoptosis in refractory anaemia with ringed sideroblasts is initiated at the stemcell level and associated with increased activation of caspases. Br. J. Haematol. 2001, 112, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Tehranchi, R.; Fadeel, B.; Forsblom, A.M. Granulocyte colonystimulating factor inhibits spontaneous cytochrome c release and mitochondria-dependent apoptosis of myelodysplastic syndrome hematopoietic progenitors. Blood 2003, 101, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.M.; Cory, S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007, 26, 1324–1337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Wang, Y.; Xie, H.Y.; Zheng, S.S. Calcitriol inhibits hepatocyte apoptosis in rat allograft by regulating apoptosis-associated genes. Int. Immun. Pharm. 2007, 7, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Dechao, L.; Eisaku, U.; Tsuyoshi, K.; Tetsuya, Y.; Tokio, O. Reactive species (ROS) control the expression of Bcl-2 family proteins by regulating their phosphorylation and ubiquitination. Cancer Sci. 2004, 95, 644–650. [Google Scholar]
- Salganik, R.I. The benefits and hazards of antioxidants: Controlling apoptosis and other protective mechanisms in cancer patients and the human population. J. Am. Coll. Nutr. 2001, 20, 464–472. [Google Scholar] [CrossRef]
- Singh, S.V.; Srivastava, S.K.; Choi, S.; Lew, K.L.; Antosiewicz, J.; Xiao, D.; Zeng, Y.; Watkins, S.C.; Johnson, C.S.; Trump, D.L.; et al. Sulforaphane induced cell death in human prostate cancer cells is initiated by reactive oxygen species. J. Biol. Chem. 2005, 280, 19911–19924. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.S.; Liao, Y.C.; Yu, F.Y.; Chang, C.H.; Liu, B.H. Mechanism of patulin-induced apoptosis in human leukemia cells (HL-60). Toxicol. Lett. 2008, 183, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Kei, T.; Shuuji, M.; Shin, Y.; Tadayoshi, S.; Nobuhiko, T.; Michihiko, I. Tumor necrosis factor-related apoptosis-inducing ligand 1 (TRAIL1) enhances the transition of red blood cells from the larval to adult type during metamorphosis in Xenopus. Blood 2010, 115, 850–859. [Google Scholar]
- Lv, L.; Zhou, Z.X.; Huang, X.Z.; Zhao, Y.P.; Zhang, L.; Shi, Y.X.; Sun, M.L.; Zhang, J.W. Inhibition of peptidyl-prolyl cis/trans isomerase Pin1 induces cell cycle arrest and apoptosis in vascular smooth muscle cells. Apoptosis 2010, 15, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhang, W.C.; Liu, Q.S.; Hu, J.J.; Liu, G.T.; Du, G.H. Pinocembrin prevents glutamate-induced apoptosis in SH-SY5Y neuronal cells via decrease of Bax/Bcl-2 ratio. Eur. J. Pharmacol. 2008, 591, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Du, G.J.; Qi, L.W.; Stainley, W.; Wang, C.Z.; Yuan, C.S. Hydrophobic constituents and their potential anticancer activities from Devil′s Club Oplopanax horridus. J. Ethnopharmacol. 2010, 132, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.L.; Sun, Z.H.; He, Q.Z.; Yan, F.; Wang, Y.J.; Zhang, J. Aspirin inhibits ErbB2 to induce apoptosis in cervical cancer cells. Med. Oncol. 2010, 27, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Weidemann, M.J. Further characterization of the neutrophil oxidative brust by flow cytomery. J. Immunol. Method. 1993, 162, 261–268. [Google Scholar] [CrossRef]
- Yan, X.J.; Jiang, Z.Q.; Bi, L.; Yang, Y.; Chen, W.P. Salvianolic acid A attenuates TNF-α- and D-GalN-induced ER stress-mediated and mitochondrial-dependent apoptosis by modulating Bax/Bcl-2 ratio and calcium release in hepatocyte LO2 cells. Naunyn Schmiedeberg Arch. Pharmacol. 2015, 388, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.L.; Cheng, Q.Y.; She, M.R.; Wang, Q.; Huang, X.H.; Cao, L.Q.; Fu, X.H.; Chen, J.S. Expression of Sonic Hedgehog Signaling Components in Hepatocellular Carcinoma and Cyclopamine-induced Apoptosis through Bcl-2 Downregulation in vitro. Arch. Med. Res. 2010, 41, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.Y.; Han, B.S.; Li, Z.S.; Hua, F.Y.; Huang, F.; Wei, W.; Yang, Y.; Caimin, X. Sodium selenite induces apoptosis by ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction in human acute promyelocytic leukemia NB4 cells. Apoptosis 2009, 14, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Palacios, C.; Yerbes, R.; Lopez-Rivas, A. Flavopiridol induces cellular FLICE-inhibitory protein degradation by the proteasome and promotes TRAIL-induced early signaling and apoptosis in breast tumor cells. Cancer Res. 2006, 66, 8858–8869. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.










© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Zhang, L.; Cao, Y.; Yao, W.; Tang, Y.; Ding, A. An Ingenol Derived from Euphorbia kansui Induces Hepatocyte Cytotoxicity by Triggering G0/G1 Cell Cycle Arrest and Regulating the Mitochondrial Apoptosis Pathway in Vitro. Molecules 2016, 21, 813. https://doi.org/10.3390/molecules21060813
Yan X, Zhang L, Cao Y, Yao W, Tang Y, Ding A. An Ingenol Derived from Euphorbia kansui Induces Hepatocyte Cytotoxicity by Triggering G0/G1 Cell Cycle Arrest and Regulating the Mitochondrial Apoptosis Pathway in Vitro. Molecules. 2016; 21(6):813. https://doi.org/10.3390/molecules21060813
Chicago/Turabian StyleYan, Xiaojing, Li Zhang, Yudan Cao, Weifeng Yao, Yuping Tang, and Anwei Ding. 2016. "An Ingenol Derived from Euphorbia kansui Induces Hepatocyte Cytotoxicity by Triggering G0/G1 Cell Cycle Arrest and Regulating the Mitochondrial Apoptosis Pathway in Vitro" Molecules 21, no. 6: 813. https://doi.org/10.3390/molecules21060813
APA StyleYan, X., Zhang, L., Cao, Y., Yao, W., Tang, Y., & Ding, A. (2016). An Ingenol Derived from Euphorbia kansui Induces Hepatocyte Cytotoxicity by Triggering G0/G1 Cell Cycle Arrest and Regulating the Mitochondrial Apoptosis Pathway in Vitro. Molecules, 21(6), 813. https://doi.org/10.3390/molecules21060813