Microfluidic Devices in Advanced Caenorhabditis elegans Research
Abstract
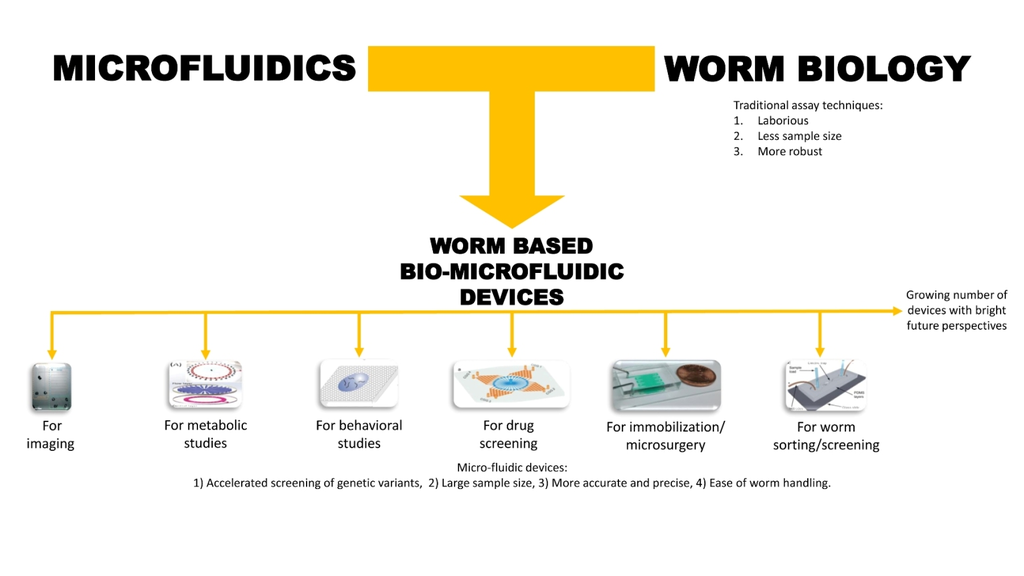
:1. Introduction
2. Basic Microfluidic Device Construction in C. elegans Research
3. Classification of Microfluidic Devices Used for C. elegans Research
4. Microfluidic Devices for Worm Applications
4.1. Immobilization and Imaging
4.2. Metabolic Studies
4.3. Behavior Analysis
4.4. Drug Screening and Toxicological Studies
4.5. Microsurgery
4.6. Worm/C. elegans Sorting
5. Challenges and Future Prospects
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Taylor, A.M.; Jeon, N.L. Micro-Scale and microfluidic devices for neurobiology. Curr. Opin. Neurobiol. 2010, 20, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Chronis, N. Worm chips: Microtools for C. elegans biology. Lab Chip 2010, 10, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Santra, T.; Tseng, F. Recent Trends on Micro/Nanofluidic Single Cell Electroporation. Micromachines 2013, 4, 333–356. [Google Scholar] [CrossRef]
- Tseng, F.G.; Santra, T.S. Micro/Nano Fluidic Devices for Single Cell Analysis; MDPI AG: Basel, Switzerland, 2015. [Google Scholar]
- Tseng, F.G.; Santra, T.S. Single Cell Analysis in Biotechnology and System Biology; MDPI AG: Basel, Switzerland, 2016. [Google Scholar]
- Tseng, F.G.; Santra, T.S. Essentials of Single-Cell Analysis, 1st ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Clausell-Tormos, J.; Lieber, D.; Baret, J.C.; El-Harrak, A.; Miller, O.J.; Frenz, L.; Blouwolff, J.; Humphry, K.J.; Koster, S.; Duan, H.; et al. Droplet-Based microfluidic platforms for the encapsulation and screening of Mammalian cells and multicellular organisms. Chem. Biol. 2008, 15, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Lin, S.C.; Kiraly, B.; Yue, H.; Li, S.; Chiang, I.K.; Shi, J.; Benkovic, S.K.; and Huang, T.J. On-Chip manipulation of single microparticles, cells, and organisms using surface acoustic waves. Proc. Natl. Acad. Sci. USA 2012, 109, 11105–11109. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Ahlawat, S.; Koushika, S.P. Simple microfluidic devices for in vivo imaging of C. elegans, Drosophila and zebrafish. J. Vis. Exp. 2012, 67. [Google Scholar] [CrossRef]
- Khare, S.M.; Awasthi, A.; Venkataraman, V.; Koushika, S.P. Colored polydimethylsiloxane micropillar arrays for high throughput measurements of forces applied by genetic model organisms. Biomicrofluidics 2015, 9. [Google Scholar] [CrossRef] [PubMed]
- Yanik, M.F.; Rohde, C.B.; Pardo-Martin, C. Technologies for micromanipulating, imaging, and phenotyping small invertebrates and vertebrates. Annu. Rev. Biomed. Eng. 2011, 13, 185–217. [Google Scholar] [CrossRef] [PubMed]
- Baker, M. Screening: The age of fishes. Nat. Methods 2011, 8, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Wlodkowic, D.; Khoshmanesh, K.; Akagi, J.; Williams, D.E.; Cooper, J.M. Wormometry-on-a-Chip: Innovative technologies for in situ analysis of small multicellular organisms. Cytometry A 2011, 79, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Crane, M.M.; Chung, K.; Stirman, J.; Lu, H. Microfluidics-Enabled phenotyping, imaging, and screening of multicellular organisms. Lab Chip 2010, 10, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Riddle, D.L.; Blumenthal, T.; Meyer, B.J.; Priess, J.R. Section I. In The Biological Model. in C. elegans II, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1997. [Google Scholar]
- Shaham, S. Methods in Cell Biology. WormBook 2006. [Google Scholar] [CrossRef]
- Fay, D. Genetic Mapping and Manipulation: Chapter 1—Introduction and Basics. Wormbook 2006. [Google Scholar] [CrossRef] [PubMed]
- Ausubel, A.; Ausubel, F.M. Caenorhabditis elegans as a host for the study of host-pathogen interactions. Curr. Opin. Microbiol. 2002, 5, 97–101. [Google Scholar]
- Hu, C.; Kearn, J.; Urwin, P.; Lilley, C.; O′Connor, V.; Holden-Dye, L.; Morgan, H. StyletChip: A microfluidic device for recording host invasion behaviour and feeding of plant parasitic nematodes. Lab Chip 2014, 14, 2447–2455. [Google Scholar] [CrossRef] [PubMed]
- Holden-Dye, L.; Walker, R.J. Anthelmintic Drugs and Nematicides: Studies in Caenorhabditis elegans. WormBook 2014. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.S.; Kuo, W.J.; Lee, C.L.; Chu, I.H.; Chen, C.S. Exercise in an electrotactic flow chamber ameliorates age-related degeneration in Caenorhabditis elegans. Sci. Rep. 2016, 6, 28064. [Google Scholar] [CrossRef] [PubMed]
- Hulme, S.E.; Whitesides, G.M. Chemistry and the worm: Caenorhabditis elegans as a platform for integrating chemical and biological research. Angew. Chem. Int. Ed. Engl. 2011, 50, 4774–4807. [Google Scholar] [CrossRef] [PubMed]
- Sivagnanam, V.; Gijs, M.A. Exploring living multicellular organisms, organs, and tissues using microfluidic systems. Chem. Rev. 2013, 113, 3214–3247. [Google Scholar] [CrossRef] [PubMed]
- Bakhtina, N.A.; Korvink, J.G. Microfluidic laboratories for C. elegans enhance fundamental studies in biology. RSC Adv. 2014, 4, 4691–4709. [Google Scholar] [CrossRef]
- San-Miguel, A.; Lu, H. Microfluidics as a Tool for C. elegans Research. WormBook 2013. [Google Scholar] [CrossRef] [PubMed]
- Lockery, S.R.; Lawton, K.J.; Doll, J.C.; Faumont, S.; Coulthard, S.M.; Thiele, T.R.; Chronis, N.; McCormick, K.E.; Goodman, M.B.; and Pruitt, B.L. Artificial dirt: Microfluidic substrates for nematode neurobiology and behavior. J. Neurophysiol. 2008, 99, 3136–3143. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zare, R.N. Perforated membrane method for fabricating three-dimensional polydimethylsiloxane microfluidic devices. Lab Chip 2008, 8, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Atencia, G.A.; Atencia, J. Pneumatic valves in folded 2D and 3D fluidic devices made from plastic films and tapes. Lap Chip 2014, 14, 1665–1668. [Google Scholar]
- Wu, H.; Odom, T.W.; Chiu, D.T.; Whitesides, G.M. Fabrication of complex three dimensional microchannel systems in PDMS. J. Am. Chem. Soc. 2002, 125, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Dafu, C.; Xia, S.; Cui, Z. An Integrated biochip design and fabrication. In Proceedings of SPIE, Nano- and Microtechnology: Materials, Processes, Packaging, and Systems, 4936; The International Society for Optical Engineering: Melbourne, Australia, 2002. [Google Scholar]
- Ren, K.; Zhou, J.; Wu, H. Materials for microfluidic chip fabrication. Acc. Chem. Res. 2012, 46, 2396–2406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, J.; Wang, L.; Xiao, K.; Wen, W. A simple method for fabricating multi-layer PDMS structures for 3D microfluidic chips. Lab Chip 2010, 10, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T. PDMS-Based microfluidic devices for biomedical applications. Microelectron. Eng. 2002, 61–62, 907–914. [Google Scholar] [CrossRef]
- Turek, M.; Besseling, J.; Bringmann, H. Agarose Microchambers for Long-term Calcium Imaging of Caenorhabditis elegans. J. Vis. Exp. 2015, 100, e52742. [Google Scholar] [CrossRef] [PubMed]
- Green, R.A.; Audhya, A.; Pozniakovsky, A.; Dammermann, A.; Pemble, H.; Monen, J.; Portier, N.; Hyman, A.; Desai, A.; Oegema, K. Expression and Imaging of Fluorescent Proteins in the C. elegans Gonad and Early Embryo. Methods Cell Biol. 2008, 85, 179–218. [Google Scholar] [PubMed]
- Chokshi, T.V.; Ben-Yakar, A.; Chronis, N. CO2 and compressive immobilization of C. elegans on-chip. Lab Chip 2009, 9, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Ahlawat, S.; Rau, K.; Venkataraman, V.; Koushika, S.P. Imaging in vivo neuronal transport in genetic model organisms using microfluidic devices. Traffic 2011, 12, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Aubry, G.; Zhan, M.; Lu, H. Hydrogel-Droplet microfluidic platform for high-resolution imaging and sorting of early larval Caenorhabditis elegans. Lab Chip 2015, 15, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Cornaglia, M.; Mouchiroud, L.; Marette, A.; Narasimhan, S.; Lehnert, T.; Jovaisaite, V.; Auwerx, J.; Gijs, M.A. An automated microfluidic platform for C. elegans embryo arraying, phenotyping, and long-term live imaging. Sci. Rep. 2015, 5, 10192. [Google Scholar] [CrossRef] [PubMed]
- Hulme, S.E.; Shevkoplyas, S.S.; Apfeld, J.; Fontana, W.; Whitesides, G.M. A microfabricated array of clamps for immobilizing and imaging C. elegans. Lab Chip 2007, 7, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kin, S. A.; Coakley, S.; Mugno, P.; Hammarlund, M.; Hilliard, M.A.; Lu, H. A multi-channel device for high-density target-selective stimulation and long-term monitoring of cells and subcellular features in C. elegans. Lab Chip 2014, 14, 4513–1522. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, S.; Wang, Y.; Parhar, R.S.; Collison, K.S.; Conca, W.; Al-Mohanna, F.; Gaugler, R.A. C. elegans model to study human metabolic regulation. Nutr. Metab. 2013, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Yin, F.; Wang, L.; Wei, W.; Jiang, L.; Qin, J. Modeling type 2 diabetes-like hyperglycemia in C. elegans on a microdevice. Integr. Biol. 2016, 8, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.C. Behavior. WormBook 2006. [Google Scholar] [CrossRef]
- Rezai, P.; Siddiqui, A.; Selvaganapathy, P.R.; Gupta, B.P. Electrotaxis of Caenorhabditis elegans in a microfluidic environment. Lab Chip 2010, 10, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Rezai, P.; Salam, S.; Selvaganapathy, P. R.; Gupta, B.P. Microfluidic-based electrotaxis for on-demand quantitative analysis of Caenorhabditis elegans’ locomotion. J. Vis. Exp. 2013, 75, e50226. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lee, I.; Jeon, T. J.; Kim, S.M. Micro-/Nanofluidic device for tunable generation of a concentration gradient: Application to Caenorhabditis elegans chemotaxis. Anal. Bioanal. Chem. 2014, 406, 2679–2686. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Ye, J.; Tan, H.; Ge, A.; Tang, L.; Feng, X.; Du, W.; Liu, B.F. Quantitative analysis of Caenorhabditis elegans chemotaxis using a microfluidic device. Anal. Chim. Acta 2015, 887, 155–162. [Google Scholar] [CrossRef] [PubMed]
- McCormick, K.E.; Gaertner, B.E.; Sottile, M.; Phillips, P.C.; Lockery, S.R. Microfluidic devices for analysis of spatial orientation behaviors in semi-restrained Caenorhabditis elegans. PLoS ONE 2011, 6, e25710. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Tang, L.; Xia, Y.; Hu, L.; Feng, X.; Du, W.; Liu, B.F. Stress response of Caenorhabditis elegans induced by space crowding in a micro-column array chip. Integr. Biol. 2013, 5, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, Z.J.; Wu, Y.Q.; Qin, L.W.; Li, Z.Y.; Wu, Z.X. Off-Response in ASH neurons evoked by CuSO4 requires the TRP channel OSM-9 in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2015, 461, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Margie, O.; Palmer, C.; Chin-Sang, I. C. elegans chemotaxis assay. J. Vis. Exp. 2013, 74, e50069. [Google Scholar] [PubMed]
- Hwang, H.; Barnes, D.E.; Matsunaga, Y.; Benian, G.M.; Ono, S.; Lu, H. Muscle contraction phenotypic analysis enabled by optogenetics reveals functional relationships of sarcomere components in Caenorhabditis elegans. Sci. Rep. 2016, 6, 19900. [Google Scholar] [CrossRef] [PubMed]
- Sonnhammer, E.L.; Durbin, R. Analysis of protein domains families in Caenorhabditis elegans. Genomics 1997, 46, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Ralf Baumeister, L.G. The worm in us—Caenorhabditis elegans as a model of human disease. Trends Biotechnol. 2002, 40, 147–148. [Google Scholar] [CrossRef]
- Carr, J.A.; Parashar, A.; Gisbon, R.; Robertson, A.P.; Martin, R.J.; Pandey, S. A microfluidic platform for high-sensitivity, real-time drug screening on C. elegans and parasitic nematodes. Lab Chip 2011, 11, 2385–2396. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.; Zhan, M.; Srinivasan, J.; Sternberg, P.W.; Gong, E.; Schroeder, F.C.; Lu, H. Microfluidic chamber arrays for whole-organism behavior-based chemical screening. Lab Chip 2011, 11, 3689–3697. [Google Scholar] [CrossRef] [PubMed]
- Lycke, R.; Parashar, A.; Pandey, S. Microfluidics-Enabled method to identify modes of Caenorhabditis elegans paralysis in four anthelmintics. Biomicrofluidics 2013, 7, 064103. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wen, H.; Lu, Y.; Shi, Y.; Lin, B.; Qin, J. Droplet microfluidics for characterizing the neurotoxin-induced responses in individual Caenorhabditis elegans. Lab Chip 2010, 10, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Lockery, S.R.; Hulme, S.E.; Roberts, W.M.; Robinson, K.J.; Laromaine, A.; Lindsay, T.H.; Whitesides, G.M.; Weeks, J.C. A microfluidic device for whole-animal drug screening using electrophysiological measures in the nematode C. elegans. Lab Chip 2012, 12, 2211–2220. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Gao, X.; Qin, J. Probing the anti-aging role of polydatin in Caenorhabditis elegans on a chip. Integr. Biol. 2014, 6, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Z.; Ching, P.; Shi, Q.; Li, X. An integrated microfluidic platform for evaluating in vivo antimicrobial activity of natural compounds using a whole-animal infection model. Lab Chip 2013, 13, 3373–3382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, Y.B.; He, Q.D.; Qin, J.; Yu, Y.Y.; Li, X.C.; Zhang, L.; Yao, M.C.; Liu, J.S.; Chen, Z.G. Microfluidic platform integrated with worm-counting setup for assessing manganese toxicity. Biomicrofluidics 2014, 8, 054110. [Google Scholar] [CrossRef] [PubMed]
- Yanik, M.F.; Cinar, H.; Cinar, H.N.; Chisholm, A.D.; Jin, Y.S.; Ben-Yakar, A. Neurosurgery: Functional regeneration after laser axotomy. Nature 2004, 432, 822. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.X.; Bourgeois, F.; Chokshi, T.; Durr, N.J.; Hilliard, M.A.; Chronis, N.; Ben-Yaker, A. Femtosecond laser nanoaxotomy lab-on-a-chip for in vivo nerve regeneration studies. Nat. Methods 2008, 5, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Gokce, S.K.; Guo, S.X.; Ghorashian, N.; Everett, W.N.; Jarrell, T.; Kottek, A.; Bovik, A.C.; Ben-Yakar, A. A fully automated microfluidic femtosecond laser axotomy platform for nerve regeneration studies in C. elegans. PLoS ONE 2014, 9, e113917. [Google Scholar] [CrossRef] [PubMed]
- Crane, M.M.; Chung, K.; Lu, H. Computer-Enhanced high-throughput genetic screens of C. elegans in a microfluidic system. Lab Chip 2009, 9, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, Z.; Yang, F.; Wang, S.; Hou, F. A microfluidic device for rapid screening of chemotaxis-defective Caenorhabditis elegans mutants. Biomed. Microdevices 2013, 15, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Casadevall i Solvas, X.; Geier, F.M.; Leroi, A.M.; Bundy, J.G.; Edel, J.B.; DeMello, A.J. High-Throughput age synchronisation of Caenorhabditis elegans. Chem. Commun. 2011, 47, 9801–9803. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Zhuo, W.; Liang, Q.; McGrath, P.T.; Lu, H. A high-throughput device for size based separation of C. elegans developmental stages. Lab Chip 2014, 14, 1746–1752. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Cornaglia, M.; Lehnert, T.; Gijs, M.A. Versatile size-dependent sorting of C. elegans nematodes and embryos using a tunable microfluidic filter structure. Lab Chip 2016, 16, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhou, J.; Raizen, D.M.; Bau, H.H. High-Throughput, motility-based sorter for microswimmers such as C. elegans. Lab Chip 2015, 15, 2790–2798. [Google Scholar] [CrossRef] [PubMed]
- Rezai, P.; Salam, S.; Selvaganapathy, P.R.; Gupta, B.P. Electrical sorting of Caenorhabditis elegans. Lab Chip 2012, 12, 1831–1840. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Dilon, J.; Kearn, J.; Murray, C.; O’Connor, V.; Holden-Dye, L.; Morgan, H. NeuroChip: A microfluidic electrophysiological device for genetic and chemical biology screening of Caenorhabditis elegans adult and larvae. PLoS ONE 2013, 8, e64297. [Google Scholar] [CrossRef] [PubMed]











© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muthaiyan Shanmugam, M.; Subhra Santra, T. Microfluidic Devices in Advanced Caenorhabditis elegans Research. Molecules 2016, 21, 1006. https://doi.org/10.3390/molecules21081006
Muthaiyan Shanmugam M, Subhra Santra T. Microfluidic Devices in Advanced Caenorhabditis elegans Research. Molecules. 2016; 21(8):1006. https://doi.org/10.3390/molecules21081006
Chicago/Turabian StyleMuthaiyan Shanmugam, Muniesh, and Tuhin Subhra Santra. 2016. "Microfluidic Devices in Advanced Caenorhabditis elegans Research" Molecules 21, no. 8: 1006. https://doi.org/10.3390/molecules21081006
APA StyleMuthaiyan Shanmugam, M., & Subhra Santra, T. (2016). Microfluidic Devices in Advanced Caenorhabditis elegans Research. Molecules, 21(8), 1006. https://doi.org/10.3390/molecules21081006