Evaluation of the Enantiomer Specific Biokinetics and Radiation Doses of [18F]Fluspidine—A New Tracer in Clinical Translation for Imaging of σ1 Receptors
Abstract
:1. Introduction
2. Results
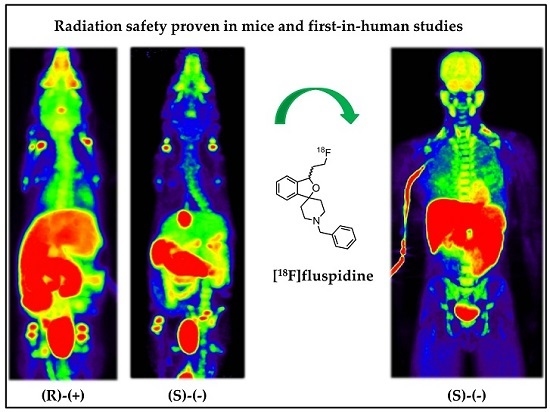
2.1. Human Dosimetry Estimation from Small Animal PET/MRI and Biodistribution Studies
2.2. Human Dosimetry from the First-in-Human Study
3. Discussion
4. Materials and Methods
4.1. Synthesis of [18F]Fluspidine
4.2. Preclinical Dosimetry Studies
4.2.1. Ex Vivo Biodistribution Study (Organ Harvesting Method)
4.2.2. In Vivo Imaging Based Study (Imaging Method)
4.3. First-in-Human Dosimetry Study (Imaging Method)
4.4. Image Reconstruction and Analysis of the Preclinical and Clinical Data
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martin, W.R.; Eades, C.; Thompson, J.; Huppler, R.; Gilbert, P. The effects of morphine-and nalorphine-like drugs in the nondependent and morphine-dependent chronic spinal dog. J. Pharmacol. Exp. Ther. 1976, 197, 517–532. [Google Scholar] [PubMed]
- Takebayashi, M.; Hayashi, T.; Su, T.-P. A perspective on the new mechanism of antidepressants: Neuritogenesis through sigma-1 receptors. Pharmacopsychiatry 2004, 37, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K. Sigma-1 receptor chaperone and brain-derived neurotrophic factor: Emerging links between cardiovascular disease and depression. Prog. Neurobiol. 2013, 100, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K.; Ishiwata, K. Sigma receptor ligands: Possible application as therapeutic drugs and as radiopharmaceuticals. Curr. Pharm. Des. 2006, 12, 3857–3876. [Google Scholar] [PubMed]
- Vilner, B.J.; John, C.S.; Bowen, W.D. Sigma-1 and sigma-2 receptors are expressed in a wide variety of human and rodent tumor cell lines. Cancer Res. 1995, 55, 408–413. [Google Scholar] [PubMed]
- Toyohara, J.; Sakata, M.; Ishiwata, K. Imaging of sigma1 receptors in the human brain using pet and [11C] SA4503. Cent. Nerv. Syst. Agents Med. Chem. 2009, 9, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, R.; Nobler, M.; Zhou, Y.; Chang, R.; Morales, O.; Kuwabawa, H.; Kumar, A.; VanHeertum, R.; Wong, D.; Sackeim, H. First evaluation of the sigma-1 receptor radioligand [18F] 1-3-fluoropropyl-4-((4-cyanophenoxy)-methyl) piperidine ([18F] FPS) in humans. Neuroimage 2004, 22, T29–T30. [Google Scholar]
- Fischer, S.; Wiese, C.; Maestrup, E.G.; Hiller, A.; Deuther-Conrad, W.; Scheunemann, M.; Schepmann, D.; Steinbach, J.; Wünsch, B.; Brust, P. Molecular imaging of sigma receptors: Synthesis and evaluation of the potent sigma1 selective radioligand [18F]fluspidine. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Brust, P.; Deuther-Conrad, W.; Becker, G.; Patt, M.; Donat, C.K.; Stittsworth, S.; Fischer, S.; Hiller, A.; Wenzel, B.; Dukic-Stefanovic, S. Distinctive in vivo kinetics of the new σ1 receptor ligands (R)-(+)-and (S)-(–)-[18F]fluspidine in porcine brain. J. Nucl. Med. 2014, 55, 1730–1736. [Google Scholar] [CrossRef] [PubMed]
- Holl, K.; Falck, E.; Köhler, J.; Schepmann, D.; Humpf, H.U.; Brust, P.; Wünsch, B. Synthesis, characterization, and metabolism studies of fluspidine enantiomers. Chem. Med. Chem. 2013, 8, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Sakata, M.; Oda, K.; Toyohara, J.; Ishii, K.; Nariai, T.; Ishiwata, K. Direct comparison of radiation dosimetry of six pet tracers using human whole-body imaging and murine biodistribution studies. Ann. Nucl. Med. 2013, 27, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Ishiwata, K.; Shimada, Y.; Kimura, Y.; Kobayashi, T.; Matsuno, K.; Homma, Y.; Senda, M. Preclinical evaluation of [11C]-SA4503: Radiation dosimetry,in vivo selectivity and pet imaging of sigma1 receptors in the cat brain. Ann. Nucl. Med. 2000, 14, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, C.C.; Garcia, A.; Mirbolooki, M.R.; Pan, M.-L.; Mukherjee, J. Evaluation of [18F]nifene biodistribution and dosimetry based on whole-body pet imaging of mice. Nucl. Med. Biol. 2013, 40, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Bretin, F.; Mauxion, T.; Warnock, G.; Bahri, M.A.; Libert, L.; Lemaire, C.; Luxen, A.; Bardiès, M.; Seret, A.; Plenevaux, A. Hybrid micropet imaging for dosimetric applications in mice: Improvement of activity quantification in dynamic micropet imaging for accelerated dosimetry applied to 6-[18F]fluoro-l-dopa and 2-[18F]fluoro-l-tyrosine. Mol. Imaging Biol. 2013, 16, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Bretin, F.; Warnock, G.; Bahri, M.A.; Libert, L.; Lemaire, C.; Phillips, C.; Seret, A.; Luxen, A.; Plenevaux, A. Dosimetry for 6-[18F] fluoro-l-dopa in humans based on in vivo micropet scans and ex vivo tissue distribution in mice. In Proceedings of the World Molecular Imaging Congress, Dublin, Ireland, 4–8 September 2012.
- Zanotti-Fregonara, P.; Innis, R.B. Suggested pathway to assess radiation safety of 11C-labeled pet tracers for first-in-human studies. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 544–547. [Google Scholar] [CrossRef] [PubMed]
- Parsey, R.V.; Sokol, L.O.; Bélanger, M.-J.; Kumar, J.S.D.; Simpson, N.R.; Wang, T.; Pratap, M.; Van Heertum, R.L.; John Mann, J. Amyloid plaque imaging agent [C-11]-6-OH-BTA-1: Biodistribution and radiation dosimetry in baboon. Nucl. Med. Commun. 2005, 26, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Sprague, D.R.; Fujita, M.; Ryu, Y.H.; Liow, J.-S.; Pike, V.W.; Innis, R.B. Whole-body biodistribution and radiation dosimetry in monkeys and humans of the phosphodiesterase 4 radioligand [11C](R)-rolipram: Comparison of two-dimensional planar, bisected and quadrisected image analyses. Nucl. Med. Biol. 2008, 35, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Liow, J.-S.; Kreisl, W.; Zoghbi, S.S.; Lazarova, N.; Seneca, N.; Gladding, R.L.; Taku, A.; Herscovitch, P.; Pike, V.W.; Innis, R.B. P-glycoprotein function at the blood–brain barrier imaged using [11C]-n-desmethyl-loperamide in monkeys. J. Nucl. Med. 2009, 50, 108–115. [Google Scholar] [CrossRef] [PubMed]
- McParland, B.J. Nuclear Medicine Radiation Dosimetry: Advanced Theoretical Principles, 1st ed.; Springer: London, UK, 2010. [Google Scholar]
- Guidance for Industry, Developing Medical Imaging Drug and Biological Products, Part 1: Conducting Safety Assessments; iii B. Table 1; U.S. Department of Health and Human Services, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER): Rockville, MD, USA, 2004; p. 7.
- A Guideline on Summary of Product Characteristics (SmPC). European Commission, Consumer Goods, Pharmaceuticals, 2009; 2, p. 27. Avaialble online: http://ec.europa.eu/health/files/eudralex/vol-2/c/smpc_guideline_rev2_en.pdf (accessed on 31 August 2016).
- Radiopharmaceuticals /iii/3936/89. European Commission, E.A.I.D.-G., Consumer Goods, Pharmaceuticals, 1990; pp. 182–183. Avaialble online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003653.pdf (accessed on 31 August 2016).
- Sattler, B.; Kranz, M.; Starke, A.; Wilke, S.; Donat, C.K.; Deuther-Conrad, W.; Patt, M.; Schildan, A.; Patt, J.; Smits, R. Internal dose assessment of (–)-[18F]flubatine, comparing animal model datasets of mice and piglets with first-in-human results. J. Nucl. Med. 2014, 55, 1885–1892. [Google Scholar] [CrossRef] [PubMed]
- Kranz, M.; Sattler, B.; Tiepolt, S.; Wilke, S.; Deuther-Conrad, W.; Donat, C.K.; Fischer, S.; Patt, M.; Schildan, A.; Patt, J.; et al. Radiation dosimetry of the α4β2 nicotinic receptor ligand (+)-[18F]flubatine, comparing preclinical pet/mri and pet/ct to first-in-human pet/ct results. EJNMMI Phys. 2016. under review. [Google Scholar]
- The International Commission on Radiological Protection. ICRP Publication 60: 1990 Recommendations of the International Commission on Radiological Protection; Pergamon Press: New York, NY, USA, 1991. [Google Scholar]
- Valentin, J. The 2007 Recommendations of the International Commission on Radiological Protection; Elsevier: Oxford, UK, 2007. [Google Scholar]
- Takano, A.; Gulyás, B.; Varrone, A.; Karlsson, P.; Sjoholm, N.; Larsson, S.; Jonsson, C.; Odh, R.; Sparks, R.; Al Tawil, N.; et al. Biodistribution and radiation dosimetry of the 18 kda translocator protein (tspo) radioligand [18F]FEDAA1106: A human whole-body pet study. Eur. J. Nucl. Med. Mol. 2011, 38, 2058–2065. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Wang, M.; Tang, X.; Luo, L.; Gan, M. Pharmacokinetics and radiation dosimetry estimation of o-(2-[18F] fluoroethyl)-l-tyrosine as oncologic pet tracer. Appl. Radiat. Isot. 2003, 58, 219–225. [Google Scholar] [CrossRef]
- Pauleit, D.; Floeth, F.; Herzog, H.; Hamacher, K.; Tellmann, L.; Müller, H.-W.; Coenen, H.H.; Langen, K.-J. Whole-body distribution and dosimetry of o-(2-[18F] fluoroethyl)-l-tyrosine. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Bottlaender, M.; Valette, H.; Roumenov, D.; Dollé, F.; Coulon, C.; Ottaviani, M.; Hinnen, F.; Ricard, M. Biodistribution and radiation dosimetry of 18F-fluoro-a-85380 in healthy volunteers. J. Nucl. Med. 2003, 44, 596–601. [Google Scholar] [PubMed]
- Valentin, D.J. 3. Recalculated dose data for 19 frequently used radiopharmaceuticals from ICRP publication 53. Ann. ICRP 1998, 28, 47–83. [Google Scholar] [CrossRef]
- Stabin, M.G.; Sparks, R.B.; Crowe, E. Olinda/exm: The second-generation personal computer software for internal dose assessment in nuclear medicine. J. Nucl. Med. 2005, 46, 1023–1027. [Google Scholar] [PubMed]
- Kirschner, A.S.; Ice, R.D.; Beierwaltes, W. Radiation dosimetry of 131I-19-iodocholesterol: The pitfalls of using tissue concentration data—Reply. J. Nucl. Med. 1975, 16, 248–249. [Google Scholar]
- Alkire, M.T.; Haier, R.J.; Shah, N.K.; Anderson, C.T. Positron emission tomography study of regional cerebral metabolism in humans during isoflurane anesthesia. Anesthesiology 1997, 86, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Toyama, H.; Ichise, M.; Liow, J.-S.; Vines, D.C.; Seneca, N.M.; Modell, K.J.; Seidel, J.; Green, M.V.; Innis, R.B. Evaluation of anesthesia effects on [18F]FDG uptake in mouse brain and heart using small animal pet. J. Nucl. Med. Biol. 2004, 31, 251–256. [Google Scholar] [CrossRef]
- Maurice, T.; Su, T.-P. The pharmacology of sigma-1 receptors. Pharmacol. Ther. 2009, 124, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F.; Marthi, K.; Munk, O.L.; Cumming, P.; Hansen, S.B.; Jakobsen, S. PET neuroimaging of [11C]mirtazapine enantiomers in pigs. Eur. Neuropsychopharmacol. 2006, 16, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.A.; He, H.; Pham-Huy, C. Chiral drugs: An overview. Int. J. Biomed. Sci. 2006, 2, 85–100. [Google Scholar] [PubMed]
- Bothschafter, S. Auswirkungen der Narkose mit Isofluran auf Die Kognitive Leistungsfähigkeit von 3 Monate Alten, Transgenen Alzheimer-Mäusen und Ihren Gesunden Wurfgeschwistern; Ludwig-Maximilians-Universität München: München, Germany, 2005. [Google Scholar]
- Matsuura, S.; Downie, J. Effect of anesthetics on reflex micturition in the chronic cannula-implanted rat. Neurourol. Urodyn. 2000, 19, 87–99. [Google Scholar] [CrossRef]
- Yaksh, T.; Durant, P.; Brent, C. Micturition in rats: A chronic model for study of bladder function and effect of anesthetics. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1986, 251, R1177–R1185. [Google Scholar]
- Smith, P.P.; DeAngelis, A.M.; Kuchel, G.A. Evidence of central modulation of bladder compliance during filling phase. Neurourol. Urodyn. 2012, 31, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Große Maestrup, E.; Fischer, S.; Wiese, C.; Schepmann, D.; Hiller, A.; Deuther-Conrad, W.; Steinbach, J.; Wünsch, B.; Brust, P. Evaluation of spirocyclic 3-(3-fluoropropyl)-2-benzofurans as σ1 receptor ligands for neuroimaging with positron emission tomography. J. Med. Chem. 2009, 52, 6062–6072. [Google Scholar] [CrossRef] [PubMed]
- Kortekaas, R.; Maguire, R.P.; van Waarde, A.; Leenders, K.L.; Elsinga, P.H. Despite irreversible binding, pet tracer [11C]-SA5845 is suitable for imaging of drug competition at sigma receptors—The cases of ketamine and haloperidol. Neurochem. Int. 2008, 53, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Van Waarde, A.; Jager, P.L.; Ishiwata, K.; Dierckx, R.A.; Elsinga, P.H. Comparison of sigma-ligands and metabolic PET tracers for differentiating tumor from inflammation. J. Nucl. Med. 2006, 47, 150–154. [Google Scholar] [PubMed]
- Maisonial-Besset, A.; Funke, U.; Wenzel, B.; Fischer, S.; Holl, K.; Wünsch, B.; Steinbach, J.; Brust, P. Automation of the radiosynthesis and purification procedures for [18F] fluspidine preparation, a new radiotracer for clinical investigations in pet imaging of σ1 receptors in brain. Appl. Radiat. Isot. 2014, 84, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Deuther-Conrad, W.; Maisonial, A.; Patt, M.; Stittsworth, S.; Becker, G.; Habermann, B.; Holl, K.; Schepmann, D.; Funke, U.; Donat, C. Discovery of enantioselective suitability of (R)-(+)-and (S)-(−)-[F-18] fluspidine for sigma 1 receptor imaging. J. Label. Compd. Radiopharm. 2013, 56, S55. [Google Scholar]
- Hofmann, M.; Steinke, F.; Scheel, V.; Charpiat, G.; Farquhar, J.; Aschoff, P.; Brady, M.; Schölkopf, B.; Pichler, B.J. Mri-based attenuation correction for pet/mri: A novel approach combining pattern recognition and atlas registration. J. Nucl. Med. 2008, 49, 1875–1883. [Google Scholar] [CrossRef]
- Stabin, M.G. Fundamentals of Nuclear Medicine Dosimetry, 1st ed.; Springer: London, UK, 2008. [Google Scholar]
- Sparks, R.; Aydogan, B. Comparison of the effectiveness of some common animal data scaling techniques in estimating human radiation dose. In Sixth International Radiopharmaceutical Dosimetry Symposium; Oak Ridge Associated Universities: Oak Ridge, TN, USA, 1999; pp. 705–716. [Google Scholar]
- Bolch, W.E.; Eckerman, K.F.; Sgouros, G.; Thomas, S.R. Mird pamphlet no. 21: A generalized schema for radiopharmaceutical dosimetry—Standardization of nomenclature. J. Nucl. Med. 2009, 50, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Loevinger, R.; Budinger, T.F.; Watson, E.E. MIRD primer for absorbed dose calculations. Soc. Nucl. Med. 1988, 14, 723–724. [Google Scholar]
- Stabin, M. Nuclear medicine dosimetry. Phys. Med. Biol. 2006, 51, R187. [Google Scholar] [CrossRef] [PubMed]
- Menzel, H.; Clement, C.; DeLuca, P. Icrp publication 110. Realistic reference phantoms: An icrp/icru joint effort. A report of adult reference computational phantoms. Ann. ICRP 2009, 39, 1–164. [Google Scholar] [PubMed]
- Sample Availability: Samples of the compounds are available from the authors.

| Target Organ | (S)-(−)-[18F]Fluspidine | (R)-(+)-[18F]Fluspidine | ||||||
|---|---|---|---|---|---|---|---|---|
| OD | SD | ED Contr. | SD | OD | SD | ED Contr. | SD | |
| Adrenals | 10.50 | 0.74 | 0.09 | 0.01 | 11.00 | 1.55 | 0.09 | 0.01 |
| Brain | 10.10 | 2.34 | 0.10 | 0.02 | 13.20 | 1.19 | 0.13 | 0.01 |
| Breasts | 5.93 | 0.10 | 0.71 | 0.01 | 6.19 | 1.77 | 0.74 | 0.21 |
| Gallbladder Wall | 25.60 | 9.57 | 0.22 | 0.08 | 30.10 | 11.90 | 0.26 | 0.10 |
| LLI Wall | 14.00 | 1.48 | 0.84 | 0.09 | 13.80 | 1.39 | 0.83 | 0.08 |
| Small Intestine | 23.10 | 3.22 | 0.20 | 0.03 | 22.60 | 1.92 | 0.20 | 0.02 |
| Stomach Wall | 10.50 | 0.60 | 1.26 | 0.07 | 12.70 | 1.10 | 1.52 | 0.13 |
| ULI Wall | 20.50 | 4.96 | 1.23 | 0.30 | 25.60 | 2.19 | 1.54 | 0.13 |
| Heart Wall | 9.85 | 0.60 | 0.08 | 0.01 | 10.50 | 1.31 | 0.09 | 0.01 |
| Kidneys | 37.60 | 14.80 | 0.32 | 0.13 | 26.90 | 2.74 | 0.23 | 0.02 |
| Liver | 25.00 | 3.23 | 1.00 | 0.13 | 26.10 | 4.65 | 1.04 | 0.19 |
| Lungs | 10.40 | 2.30 | 1.25 | 0.28 | 10.80 | 0.89 | 1.30 | 0.11 |
| Muscle | 7.57 | 0.07 | 0.07 | 0.00 | 7.86 | 1.96 | 0.07 | 0.02 |
| Ovaries | 11.50 | 0.82 | 0.92 | 0.07 | 11.90 | 1.95 | 0.95 | 0.16 |
| Pancreas | 10.90 | 0.69 | 0.09 | 0.01 | 24.80 | 1.79 | 0.21 | 0.02 |
| Red Marrow | 10.80 | 0.37 | 1.30 | 0.04 | 12.80 | 1.27 | 1.53 | 0.15 |
| Osteogenic Cells | 12.70 | 0.13 | 0.13 | 0.00 | 14.00 | 3.18 | 0.14 | 0.03 |
| Skin | 5.61 | 0.02 | 0.06 | 0.00 | 5.82 | 1.73 | 0.06 | 0.02 |
| Spleen | 26.10 | 7.29 | 0.22 | 0.06 | 31.80 | 20.00 | 0.27 | 0.17 |
| Testes | 7.46 | 0.39 | 0.00 | 0.00 | 7.63 | 2.04 | 0.00 | 0.00 |
| Thymus | 7.19 | 0.11 | 0.06 | 0.00 | 7.52 | 2.21 | 0.06 | 0.02 |
| Thyroid | 7.61 | 1.09 | 0.30 | 0.04 | 10.10 | 0.35 | 0.41 | 0.01 |
| Urinary Bladder Wall | 58.00 | 15.90 | 2.32 | 0.64 | 55.70 | 19.30 | 2.23 | 0.77 |
| Uterus | 12.80 | 1.28 | 0.11 | 0.01 | 13.00 | 1.49 | 0.11 | 0.01 |
| Total Body | 8.68 | 0.14 | 0.00 | 0.00 | 9.13 | 1.67 | 0.00 | 0.00 |
| ED | 12.9 | 0.4 | 14.0 | 0.5 | ||||
| ED ICRP 60 | 14.8 | 1.7 | 15.2 | 1.9 | ||||
| Target Organ | (S)-(−)-[18F]Fluspidine | (R)-(+)-[18F]Fluspidine | ||
|---|---|---|---|---|
| OD | ED Contr. | OD | ED Contr. | |
| Adrenals | 36.0 | 0.3 | 18.6 | 0.2 |
| Brain | 12.4 | 0.1 | 12.6 | 0.1 |
| Breasts | 11.2 | 1.3 | 11.3 | 1.4 |
| Gallbladder Wall | 15.5 | 0.1 | 14.0 | 0.1 |
| LLI Wall | 19.0 | 1.1 | 16.4 | 1.0 |
| Small Intestine | 31.9 | 0.3 | 25.1 | 0.2 |
| Stomach Wall | 14.8 | 1.8 | 14.3 | 1.7 |
| ULI Wall | 33.3 | 2.0 | 25.6 | 1.5 |
| Heart Wall | 17.9 | 0.2 | 22.3 | 0.2 |
| Kidneys | 35.6 | 0.3 | 27.6 | 0.2 |
| Liver | 12.5 | 0.5 | 10.3 | 0.4 |
| Lungs | 30.5 | 3.7 | 45.5 | 5.5 |
| Muscle | 7.2 | 0.1 | 7.1 | 0.1 |
| Ovaries | 17.0 | 1.4 | 24.9 | 2.0 |
| Pancreas | 26.2 | 0.2 | 21.7 | 0.2 |
| Red Marrow | 13.6 | 1.6 | 13.5 | 1.6 |
| Osteogenic Cells | 19.6 | 0.2 | 19.1 | 0.2 |
| Skin | 9.1 | 0.1 | 8.7 | 0.1 |
| Spleen | 17.6 | 0.2 | 17.2 | 0.1 |
| Testes | 11.2 | - | 10.8 | - |
| Thymus | 12.0 | 0.1 | 19.3 | 0.2 |
| Thyroid | 11.7 | 0.5 | 11.5 | 0.5 |
| Urinary Bladder Wall | 13.9 | 0.6 | 20.2 | 0.8 |
| Uterus | 15.9 | 0.1 | 14.9 | 0.1 |
| Total Body | 12.5 | - | 12.2 | - |
| ED | 16.7 | 18.4 | ||
| ED ICRP 60 | 17.3 | 20.1 | ||
| Target Organ | (S)-(−)-[18F]Fluspidine | |||
|---|---|---|---|---|
| OD | SD | ED Contr. | SD | |
| Adrenals | 15.3 | 1.1 | 0.1 | 0.0 |
| Brain | 22.6 | 4.2 | 0.2 | 0.1 |
| Breasts | 6.5 | 0.5 | 0.8 | 0.1 |
| Gallbladder Wall | 60.7 | 10.6 | 0.5 | 0.1 |
| LLI Wall | 16.6 | 5.1 | 1.0 | 0.3 |
| Small Intestine | 56.9 | 10.6 | 0.5 | 0.1 |
| Stomach Wall | 31.5 | 3.3 | 3.8 | 0.4 |
| ULI Wall | 24.3 | 5.2 | 1.5 | 0.3 |
| Heart Wall | 17.7 | 1.3 | 0.2 | 0.0 |
| Kidneys | 31.1 | 5.2 | 0.3 | 0.0 |
| Liver | 76.0 | 17.7 | 3.0 | 0.4 |
| Lungs | 28.2 | 2.9 | 3.4 | 0.3 |
| Muscle | 7.8 | 0.5 | 0.1 | 0.0 |
| Ovaries | 13.8 | 1.0 | 1.0 | 0.5 |
| Pancreas | 15.9 | 0.7 | 0.1 | 0.0 |
| Red Marrow | 23.2 | 2.2 | 2.8 | 0.1 |
| Osteogenic Cells | 18.0 | 1.6 | 0.2 | 0.0 |
| Skin | 5.3 | 0.5 | 0.1 | 0.0 |
| Spleen | 24.0 | 4.2 | 0.2 | 0.0 |
| Testes | 8.0 | 2.6 | 0.8 | 0.4 |
| Thymus | 7.5 | 0.7 | 0.1 | 0.0 |
| Thyroid | 8.4 | 1.4 | 0.3 | 0.1 |
| Urinary Bladder Wall | 24.7 | 3.4 | 1.0 | 0.1 |
| Uterus | 13.0 | 0.7 | 0.1 | 0.1 |
| Total Body | 11.4 | 0.3 | 0.0 | 0.0 |
| ED | 21.0 | 1.3 | ||
| ED ICRP 60 | 22.1 | 0.8 | ||
| Tracer | Target Organ | Clinical (μSv/MBq) | Preclinical (μSv/MBq) | Reference |
|---|---|---|---|---|
| (S)-(−)-[18F]fluspidine | brain, tumor | 21.0 | 12.9 (mouse, imaging) 16.7 (mouse, harvesting) | this study |
| (R)-(+)-[18F]fluspidine | brain, tumor | n.a. | 14.0 (mouse, imaging) 18.4 (mouse, harvesting) | this study |
| (−)-[18F]flubatine (formerly [18F]NCFHEB) | brain | 23.4 | 12.5 (mouse) 14.7 (piglet, imaging) | [24] |
| (+)-[18F]flubatine | brain | 23.0 | 12.1 (mouse, imaging) 14.3 (piglets, imaging) | [25] |
| [18F]FEDAA1106 | brain | 36 | 21.0 (male mouse) 26.0 (female mouse) | [28] |
| [18F]FET | brain tumor | 16.5 | 9.0 | [29,30] |
| 2-[18F]F-A85380 | brain | 19.4 | n.a. | [31] |
| [18F]FDG | multiple | 19.0 | n.a. | [32] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kranz, M.; Sattler, B.; Wüst, N.; Deuther-Conrad, W.; Patt, M.; Meyer, P.M.; Fischer, S.; Donat, C.K.; Wünsch, B.; Hesse, S.; et al. Evaluation of the Enantiomer Specific Biokinetics and Radiation Doses of [18F]Fluspidine—A New Tracer in Clinical Translation for Imaging of σ1 Receptors. Molecules 2016, 21, 1164. https://doi.org/10.3390/molecules21091164
Kranz M, Sattler B, Wüst N, Deuther-Conrad W, Patt M, Meyer PM, Fischer S, Donat CK, Wünsch B, Hesse S, et al. Evaluation of the Enantiomer Specific Biokinetics and Radiation Doses of [18F]Fluspidine—A New Tracer in Clinical Translation for Imaging of σ1 Receptors. Molecules. 2016; 21(9):1164. https://doi.org/10.3390/molecules21091164
Chicago/Turabian StyleKranz, Mathias, Bernhard Sattler, Nathanael Wüst, Winnie Deuther-Conrad, Marianne Patt, Philipp M. Meyer, Steffen Fischer, Cornelius K. Donat, Bernhard Wünsch, Swen Hesse, and et al. 2016. "Evaluation of the Enantiomer Specific Biokinetics and Radiation Doses of [18F]Fluspidine—A New Tracer in Clinical Translation for Imaging of σ1 Receptors" Molecules 21, no. 9: 1164. https://doi.org/10.3390/molecules21091164
APA StyleKranz, M., Sattler, B., Wüst, N., Deuther-Conrad, W., Patt, M., Meyer, P. M., Fischer, S., Donat, C. K., Wünsch, B., Hesse, S., Steinbach, J., Brust, P., & Sabri, O. (2016). Evaluation of the Enantiomer Specific Biokinetics and Radiation Doses of [18F]Fluspidine—A New Tracer in Clinical Translation for Imaging of σ1 Receptors. Molecules, 21(9), 1164. https://doi.org/10.3390/molecules21091164