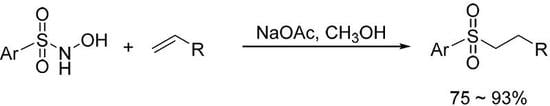
Synthesis of Alkyl Aryl Sulfones via Reaction of N-Arylsulfonyl Hydroxyamines with Electron-Deficient Alkenes
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Typical Experiment Procedure for the Reaction of N-Phenylsulfonyl Hydroxylamine (1a) with Methyl Acrylate (2a) Affording Methyl 3-(Phenylsulfonyl)propanoate (3aa) (Table 1, Entry 3)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Prilezhaeva, E.N. Sulfones and sulfoxides in the total synthesis of biologically active natural compounds. Russ. Chem. Rev. 2000, 69, 367–408. [Google Scholar] [CrossRef]
- Von Schmeling, B.; Kulka, M. Systemic fungicidal activity of 1,4-oxathiin derivatives. Science 1966, 152, 659–660. [Google Scholar] [CrossRef] [PubMed]
- Hainzl, D.; Casida, J.E. Fipronil insecticide: Novel photochemical desulfinylation with retention of neurotoxicity. Proc. Natl. Acad. Sci. USA 1996, 93, 12764–12767. [Google Scholar] [CrossRef] [PubMed]
- Sekino, K. Discovery study of new herbicides from the inhibition of photosynthetic pigments biosynthesis: Development of a new plastoquinone biosynthetic inhibitor, benzobicyclon as a herbicide. J. Pestic. Sci. 2002, 27, 388–391. [Google Scholar] [CrossRef]
- Tfelt-Hansen, P.; De Vries, P.; Saxena, P.R. Triptans in migraine. Drugs 2000, 60, 1259–1287. [Google Scholar] [CrossRef] [PubMed]
- Schellhammer, P.F. An evaluation of bicalutamide in the treatment of prostate cancer. Expert Opin. Pharmacother. 2002, 3, 1313–1328. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.M.; Qu, C.; Lynch, J.E. Zn/CuI-mediated coupling of alkyl halides with vinyl sulfones, vinyl sulfonates, and vinyl sulfonamides. J. Org. Chem. 2005, 70, 6944–6947. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, B.; Reddy, M.A.; Reddy, P.S. FeCl3/TMSCl: An effective catalytic system for the conjugate addition of sodium p-toluenesulfinate to α,β-enones. Synlett 2008, 1949–1952. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, J.; Wei, F.; Qi, Y.; Wang, H.; Liu, Z.; Lei, A. Aerobic oxysulfonylation of alkenes leading to secondary and tertiary β-hydroxysulfones. Angew. Chem. Int. Ed. 2013, 52, 7156–7159. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Liu, C.; Yang, D.; Wen, J.; You, J.; Suo, Y.; Wang, H. Copper-catalyzed direct oxysulfonylation of alkenes with dioxygen and sulfonylhydrazides leading to β-ketosulfones. Chem. Commun. 2013, 49, 10239–10241. [Google Scholar] [CrossRef] [PubMed]
- Kariya, A.; Yamaguchi, T.; Nobuta, T.; Tada, N.; Miura, T.; Itoh, A. Molecular-iodine-catalyzed aerobic oxidative synthesis of β-hydroxy sulfones from alkenes. RSC Adv. 2014, 4, 13191–13194. [Google Scholar] [CrossRef]
- Yang, Y.; Tang, L.; Zhang, S.; Guo, X.; Zha, Z.; Wang, Z. Catalyst-free sulfonylation of activated alkenes for highly efficient synthesis of mono-substituted ethyl sulfones in water. Green Chem. 2014, 16, 4106–4109. [Google Scholar] [CrossRef]
- Xiao, F.; Chen, S.; Chen, Y.; Huang, H.; Deng, G.-J. Efficient 2-sulfolmethyl quinoline formation from 2-methylquinolines and sodium sulfinates under transition-metal free conditions. Chem. Commun. 2015, 51, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N. Aerobic nickel-catalyzed hydroxysulfonylation of alkenes using sodium sulfinates. J. Org. Chem. 2015, 80, 7797–7802. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, G.; Lu, Q.; Chiang, C.-W.; Peng, P.; Zhou, J.; Lei, A. Catalyst-free difunctionalization of activated alkenes in water: Efficient synthesis of β-keto sulfides and sulfones. Chem. Eur. J. 2016, 22, 14489–14493. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Yin, P.; Chen, Y.; Deng, Y.; He, L. Preparation of α-sulfonylethanone oximes from oxidized hydroxylamine. Eur. J. Org. Chem. 2012, 2711–2714. [Google Scholar] [CrossRef]
- The known products (3aa, 3ab, 3af, 3ba, 3bb, 3ca, 3da and 3ag) were identified by their 1H-NMR and 13C-NMR, and the new products (3ac, 3ad, 3ae, 3ea, 3fa and 3cg) were characterized by their 1H-NMR, 13C-NMR and HRMS. The charts of 1H- and 13C-NMR are reported as Supplementary Materials.
- Switzer, C.H.; Miller, T.W.; Farmer, P.J.; Fukuto, J.M. Synthesis and characterization of lithium oxonitrate (LiNO). J. Inorg. Biochem. 2013, 118, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Bharadwaj, S.K.; Pandey, R.; Chaudhuri, M.K. Borax-catalyzed and pH-controlled selective oxidation of organic sulfides by H2O2: An environmentally clean protocol. Eur. J. Org. Chem. 2009, 3319–3322. [Google Scholar] [CrossRef]
- Baskin, J.M.; Wang, Z. A mild, convenient synthesis of sulfinic acid salts and sulfonamides from alkyl and aryl halides. Tetrahedron Lett. 2002, 43, 8479–8483. [Google Scholar] [CrossRef]
- Yin, C.-S.; Liu, X.-H.; Guo, W.-M.; Liu, S.-S.; Han, S.-K.; Wang, L.-S. Multi-objective modeling and assessment of partition properties: A GA-based quantitative structure-property relationship approach. Chin. J. Chem. 2003, 21, 1150–1158. [Google Scholar] [CrossRef]
- Thompson, H.P.G.; Day, G.M. Which conformations make stable crystal structures? Mapping crystalline molecular geometries to the conformational energy landscape. Chem. Sci. 2014, 5, 3173–3182. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.



| Entry | R | Solvent | Base (Equiv.) | Yield (%) b |
|---|---|---|---|---|
| 1 | COOMe | CH3CN | -- | 0 |
| 2 | CH3CN | NaOAc (1) | 39 | |
| 3 | CH3OH | NaOAc (1) | 98(93) | |
| 4 | CN | toluene | NaOAc (1) | 45 |
| 5 | CH3CN | NaOAc (1) | 53 | |
| 6 | CH3OH | NaOAc (1) | 85(80) | |
| 7 | CH3OH | NaOAc (2) | 84 | |
| 8 | CH3OH | NaOAc (0.5) | 67 | |
| 9 | CH3OH | Na2CO3 | 44 | |
| 10 | CH3OH | DABCO (1) | 0 | |
| 11 | CH3OH | DBU (1) | 0 | |
| 12 | CH3OH | pyridine | 0 | |
| 13 | CH3OH | -- | 0 |

| Entry | 1 | 2 | 3 | Yield (%) b |
|---|---|---|---|---|
| 1 | 1a |  |  | 75 |
| 2 | 1c | 2g |  | 76 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bin, Y.; Hua, R. Synthesis of Alkyl Aryl Sulfones via Reaction of N-Arylsulfonyl Hydroxyamines with Electron-Deficient Alkenes. Molecules 2017, 22, 39. https://doi.org/10.3390/molecules22010039
Bin Y, Hua R. Synthesis of Alkyl Aryl Sulfones via Reaction of N-Arylsulfonyl Hydroxyamines with Electron-Deficient Alkenes. Molecules. 2017; 22(1):39. https://doi.org/10.3390/molecules22010039
Chicago/Turabian StyleBin, Yunhui, and Ruimao Hua. 2017. "Synthesis of Alkyl Aryl Sulfones via Reaction of N-Arylsulfonyl Hydroxyamines with Electron-Deficient Alkenes" Molecules 22, no. 1: 39. https://doi.org/10.3390/molecules22010039
APA StyleBin, Y., & Hua, R. (2017). Synthesis of Alkyl Aryl Sulfones via Reaction of N-Arylsulfonyl Hydroxyamines with Electron-Deficient Alkenes. Molecules, 22(1), 39. https://doi.org/10.3390/molecules22010039