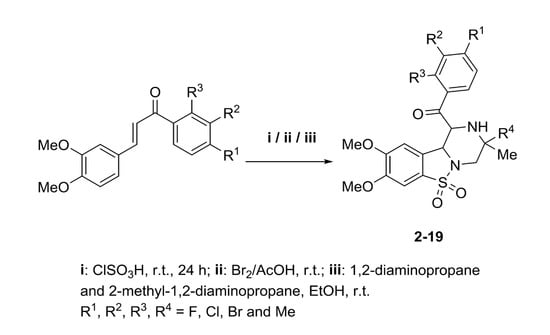
3. Experimental Section
3.1. General Information
All chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA) and were used without any further purification. Melting points were determined using a Gallenkamp melting point apparatus (Thermo Fisher Scientific, Paisley, UK) and are uncorrected. The NMR spectra were recorded using a 600 MHz spectrometer (JEOL Co. Ltd., Tokyo, Japan) with tetramethylsilane as the internal standard and solvents as indicated. Chemical shifts were measured in ppm (δ) relative to TMS (0.00 ppm). Coupling constants (J) are reported in Hertz (Hz). The following abbreviations are used to describe the signal multiplicities: s (singlet), d (doublet), t (triplet), q (quartet) and m (multiplet). LC-MS spectra were obtained with a spectrometer equipped with an Electron Spray Ionisation (ESI) source (Varian: 210 LC pumps × 2, 1200 L Quadrapole MS/MS, 410 autosampler) (Varian (now Agilent), Oxford, UK) using a gradient solvent system of A: Water/0.1% formic acid and B: acetonitrile/0.1% formic acid. Infrared spectra were recorded with a Varian 800 FT-IR spectrophotometer (Varian) as KBr discs.
3.2. Synthesis of Chalcones
The chalcones were synthesized by the well-established procedure using acetophenones and 3,4-dimethoxybenzaldehyde [
22,
24,
25,
26,
27,
28].
3.3. General Procedure for Synthesis of Chalcone Sulfonyl Chlorides
The chalcones (10 g; 0.032 mol) were added in portions to stirred chlorosulfonic acid (37.67 g; 0.32 mol) in an ice bath. After the addition was complete, the reaction mixture was left stirring at room temperature. Progress of the reaction was monitored by thin layer chromatography (TLC) (Fisher Scientific, Loughborough, UK When the reaction was complete (24 h), the mixture was poured slowly over ice to remove excess chlorosulfonic acid. The sulfonyl chlorides were filtered by suction filtration and washed with a cold water acetonitrile mixture. The resulting precipitate was considered pure enough to be used in subsequent reactions by TLC analysis.
3.4. General Procedure for the Synthesis of Dibromo Chalcone Sulfonyl Chlorides
The crude chalcone sulfonyl chloride (10 g; 0.032 mol) was added to glacial acetic acid (125 mL) with stirring. The resulting mixture was stirred at room temperature and to the stirred mixture was added bromine (20.48 g; 0.13 mol) dissolved in 50 mL glacial acetic acid. The mixture was stirred until a precipitate was formed, which was filtered and washed with cold glacial acetic acid.
3.5. General Method for Synthesis of Benzo[4,5]isothiazolo[2,3-a]pyrazin-1-yl)(phenyl) Methanones (2–10)
To a stirred solution of the chalcone dibromo sulphonyl chloride (1 g) in ethanol (25 mL), was added 1,2-diaminopropane (2 mole equivalent). The reaction mixture was warmed in a water bath for 15 min until all the solids had dissolved. The reaction mixture was allowed to cool to room temperature and the resulting solid was filtered and air dried. The product was purified by recrystallization from ethanol.
(8,9-Dimethoxy-3-methyl-6,6-dioxido-2,3,4,10b-tetrahydro-1H-benzo[4,5]isothiazolo[2,3-a]pyrazin-1-yl)(Phenyl)methanone (2). Yellow crystals; yield 29%; m.p. 219–220 °C. IR: (KBr ν cm−1) 3337 (NH); 1681 (C=O), 1287, 1176 (SO2). 1H-NMR (600 MHz, CDCl3) δ ppm 1.15 (d, J = 6.42 Hz, 3 H), 2.79–2.88 (m, 1 H), 3.14–3.36 (m, 1 H), 3.48 (s, 3 H), 3.89 (s, 3 H), 3.90–3.94 (m, 1 H), 4.42 (d, J = 10.09 Hz, 1 H), 4.64 (d, J = 10.09 Hz, 1 H), 6.40 (s, 1 H), 7.21 (s, 1 H), 7.43 (t, J = 7.79 Hz, 2 H), 7.58 (t, J = 7.34 Hz, 1 H), 7.90 (d, J = 8.25 Hz, 2 H). 13C-NMR (CDCl3) δ ppm 18.90, 47.30, 48.29, 56.02, 56.40, 58.49, 62.49, 102.81, 107.34, 126.42, 127.87, 129.07, 129.17, 134.51, 135.54, 135.51, 150.47, 152.62, 198.30. MS (ESI m/z) 402.3 [M]+. Anal. Calcd. For: C20H22N2O5S: C, 59.69; H, 5.51. Found: C, 59.35; H, 5.60.
(8,9-Dimethoxy-3-methyl-6,6-dioxido-2,3,4,10b-tetrahydro-1H-benzo[4,5]isothiazolo[2,3-a]pyrazin-1-yl)(p-tolyl)methanone (3). White crystals; yield 47%; m.p. 180–181 °C. IR: (KBr ν cm−1) 3447 (NH), 1663 (C=O), 1284, 1140 (SO2). 1H-NMR (600 MHz, CDCl3) δ ppm 1.53 (d, J = 6.19 Hz, 3 H), 2.42 (m, 3 H), 3.13 (m, 1 H), 3.50 (d, J = 14.43 Hz, 1 H), 3.77 (dd, J = 14.43, 1.37 Hz, 1 H), 3.94 (s, 3 H), 3.98 (s, 3 H), 4.29 (d, J = 11.00 Hz, 1 H), 6.80 (s, 1 H), 7.26 (m, 3 H), 7.68 (d, J = 8.25 Hz, 2 H). 13C-NMR (151 MHz, CDCl3) δ ppm 22.48, 38.76, 45.67, 55.47, 56.54, 56.67, 56.95, 102.97, 105.06, 123.14, 125.38, 126.06, 129.77, 130.15, 130.23, 133.17, 142.62, 150.81, 153.59, 166.65. MS (ESI m/z) 416.3 [M]+. Anal. Calcd. For: C21H24N2O5S: C, 60.56; H, 5.81. Found: C, 60.60; H, 5.72.
(8,9-Dimethoxy-3-methyl-6,6-dioxido-2,3,4,10b-tetrahydro-1H-benzo[4,5]isothiazolo[2,3-a]pyrazin-1-yl)(4-fluorophenyl)methanone (4). Yellow crystals; yield 30%; m.p. 181–182 °C. IR: (KBr ν cm−1) 3297 (NH), 1677 (C=O), 1290, 1144 (SO2). 1H-NMR (151 MHz, CDCl3) δ ppm 1.14 (d, J = 6.42 Hz, 3 H), 3.55 (s, 3 H), 3.89 (s, 3 H), 3.92 (dd, J = 14.67, 3.67 Hz, 1 H), 4.34 (d, J = 10.09 Hz, 1 H), 4.65 (d, J = 9.17 Hz, 1 H), 6.45 (s, 1 H), 7.10 (t, J = 8.71 Hz, 2 H), 7.21 (s, 1 H), 7.95 (m, 2 H). 13C-NMR (151 MHz, CDCl3) δ ppm 18.93, 47.40, 48.28, 56.25, 58.23, 59.07, 62.61, 102.86, 107.26, 116.21, 116.36, 126.47, 127.92, 131.92, 131.91, 150.54, 152.70, 167.37, 196.74. MS (ESI m/z) 420.2 [M]+. Anal. Calcd. For: C20H21FN2O5S: C, 57.13; H, 5.03. Found: C, 57.23; H, 4.95.
(8,9-Dimethoxy-3-methyl-6,6-dioxido-2,3,4,10b-tetrahydro-1H-benzo[4,5]isothiazolo[2,3-a]pyrazin-1-yl)(4-chlorophenyl)methanone (5). Yellow crystals; yield 43%; m.p. 204–205 °C. IR: (KBr ν cm−1) 3468 (NH), 1672 (C=O), 1284, 1142 (SO2); 1H-NMR (600 MHz, CDCl3) δ ppm 1.52 (d, J = 6.87 Hz, 3 H), 2.99 (dd, J = 14.43, 9.62 Hz, 1 H), 3.14 (dd, J = 14.78, 11.34 Hz, 1 H), 3.45 (d, J = 15.12 Hz, 1 H), 3.77 (dd, J = 14.43, 1.37 Hz, 1 H), 3.95 (m, 3 H), 3.98 (m, 3 H), 4.28 (d, J = 11.68 Hz, 1 H), 6.79 (s, 1 H), 7.25 (s, 1 H), 7.42 (m, 2 H), 7.73 (d, J = 8.25 Hz, 2 H). 13C-NMR (151 MHz, CDCl3) δ ppm 22.58, 38.68, 45.70, 55.54, 56.55, 56.66, 56.70, 102.93, 104.95, 115.69, 115.83, 126.04, 128.98, 129.03, 129.89, 136.72, 150.74, 153.53, 166.84. MS (ESI m/z) 436.2 [M]+. Anal. Calcd. For: C20H21ClN2O5S: C, 54.98; H, 4.84. Found: C, 55.13; H, 4.75.
(8,9-Dimethoxy-3-methyl-6,6-dioxido-2,3,4,10b-tetrahydro-1H-benzo[4,5]isothiazolo[2,3-a]pyrazin-1-yl)(4-bromophenyl)methanone (6). Yellow crystals; yield 48%; m.p. 204–205 °C. IR (KBr ν cm−1): 3458 (NH), 1672 (C=O), 1284, 1141 (SO2). 1H-NMR (600 MHz, CDCl3) δ ppm 1.52 (d, J = 6.87 Hz, 3 H), 2.99 (dd, J = 14.43, 9.62 Hz, 1 H), 3.14 (dd, J = 14.78, 11.34 Hz, 1 H), 3.44 (d, J = 15.12 Hz, 1 H), 3.77 (d, J = 15.12 Hz, 1 H), 3.98 (m, 3 H), 3.98 (s, 3 H), 4.28 (d, J = 11.00 Hz, 1 H), 6.78 (s, 1 H), 7.25 (s, 1 H), 7.58 (d, J = 8.94 Hz, 2 H), 7.66 (m, 2 H). 13C-NMR (151 MHz, CDCl3) δ ppm 21.44, 22.63, 38.71, 45.73, 55.65, 56.28, 56.63, 102.89, 105.01, 126.01, 126.88, 129.47, 130.12, 137.88, 140.57, 150.66, 153.49, 167.93. MS (ESI m/z) 480.4 [M]+. Anal. Calcd. For: C20H21BrN2O5S: C, 49.90; H, 4.40. Found: C, 49.85; H, 4.36.
(8,9-Dimethoxy-3-methyl-6,6-dioxido-2,3,4,10b-tetrahydro-1H-benzo[4,5]isothiazolo[2,3-a]pyrazin-1-yl)(3-chlorophenyl)methanone (7). Light yellow crystals; yield 79%; m.p. 194–195 °C. IR (KBr ν cm−1): 1691 (C=O), 1270, 1158 (SO2). 1H-NMR (600 MHz, CDCl3) δ ppm 1.53 (d, J = 6.87 Hz, 3 H), 3.00 (dd, J = 14.43, 9.62 Hz, 1 H), 3.44 (m, 1 H), 3.78 (dd, J = 14.43, 1.37 Hz, 1 H), 3.95 (s, 3 H), 3.99 (m, 3 H), 4.29 (d, J = 11.00 Hz, 1 H), 6.80 (s, 1 H), 7.25 (s, 1 H), 7.39 (m, 1 H), 7.43 (m, 1 H), 7.63 (m, 1 H), 7.78 (t, J = 1.72 Hz, 1 H). 13C-NMR (151 MHz, CDCl3) δ ppm 14.87, 41.86, 46.31, 56.54, 56.65, 56.78, 56.97, 67.41, 110.98, 111.87, 112.01, 112.30, 127.15, 129.00, 130.45, 131.31, 134.17, 134.27, 134.94, 135.44, 135.79, 149.16, 149.30, 154.75, 188.68. MS (ESI m/z) 436.2 [M]+. Anal. Calcd. For: C20H21ClN2O5S: C, 54.98; H, 4.84. Found: C, 55.23; H, 4.92.
(8,9-Dimethoxy-3-methyl-6,6-dioxido-2,3,4,10b-tetrahydro-1H-benzo[4,5]isothiazolo[2,3-a]pyrazin-1-yl)(3-bromophenyl)methanone (8). Yellow crystals; yield 50%; m.p. 189–190 °C. IR (KBr ν cm−1): 3455 (NH), 1686 (C=O), 1290, 1140 (SO2). 1H-NMR (600 MHz, CDCl3) δ ppm 1.15 (d, J = 6.42 Hz, 3 H), 2.84 (m, 1 H), 3.27 (m, 1 H), 3.48 (s, 3 H), 3.89 (m, 3 H), 4.42 (d, J = 10.09 Hz, 1 H), 4.64 (d, J = 10.09 Hz, 1 H), 6.40 (s, 1 H), 7.21 (m, 1 H), 7.43 (t, J = 7.79 Hz, 2 H), 7.58 (t, J = 7.34 Hz, 2 H). 13C-NMR (151 MHz, CDCl3) δ ppm 18.71, 47.37, 48.36, 58.32, 58.21, 58.54, 62.85, 102.91, 107.23, 123.45, 126.45, 127.78, 127.72, 127.82, 131.85, 132.20, 137.22, 150.59, 152.77, 197.14. MS (ESI m/z) 482.7 [M]+. Anal. Calcd. For: C20H21BrN2O5S: C, 49.90; H, 4.40. Found: C, 49.80; H, 4.45.
(8,9-Dimethoxy-3-methyl-6,6-dioxido-2,3,4,10b-tetrahydro-1H-benzo[4,5]isothiazolo[2,3-a]pyrazin-1-yl)(2-chlorophenyl)methanone (9). Yellow crystals; yield 46%; m.p. 215–216 °C. IR (KBr ν cm−1): 3388 (NH), 1683 (C=O), 1290, 1190 (SO2). 1H-NMR (600 MHz, CDCl3) δ ppm 1.54 (d, J = 6.87 Hz, 3 H), 3.09 (dd, J = 14.43, 8.94 Hz, 1 H), 3.15 (dd, J = 14.78, 11.34 Hz, 1 H), 3.31 (d, J = 15.12 Hz, 1 H), 3.79 (d, J = 14.43 Hz, 1 H), 3.92 (s, 3 H), 3.94 (s, 3 H), 4.07 (m, 1 H), 4.66 (d, J = 11.00 Hz, 1 H), 7.24 (s, 1 H), 7.39 (d, J = 4.81 Hz, 2 H), 7.62 (d, J = 8.25 Hz, 2 H). 13C-NMR (151 MHz, CDCl3) δ ppm 22.54, 38.59, 45.68, 55.52, 56.55, 56.66, 56.83, 76.87, 77.08, 77.29, 102.97, 104.99, 126.09, 128.30, 128.96, 129.84, 136.51, 138.94, 150.79, 153.57, 166.84, 182.68, 182.36. MS (ESI m/z) 436.5 [M]+. Anal. Calcd. For: C20H21ClN2O5S: C, 54.98; H, 4.84. Found: C, 55.10; H, 5.02.
(8,9-Dimethoxy-3-methyl-6,6-dioxido-2,3,4,10b-tetrahydro-1H-benzo[4,5]isothiazolo[2,3-a]pyrazin-1-yl)(2-bromophenyl)methanone (10). Yellow crystals; yield 75%; m.p. 195–196 °C. IR (KBr ν cm−1): 3084 (NH), 1682 (C=O), 1286, 1123 (SO2). 1H-NMR (600 MHz, CDCl3) δ ppm 1.15 (d, J = 6.42 Hz, 3 H), 3.59 (s, 3 H), 3.92 (dd, J = 14.67, 3.67 Hz, 1 H), 3.89 (s, 3 H), 3.92 (m, 1 H), 4.32 (d, J = 9.17 Hz, 1 H), 4.66 (d, J = 10.09 Hz, 1 H), 6.44 (s, 1 H), 7.21 (s, 1 H), 7.29 (t, J = 7.79 Hz, 1 H), 7.69 (d, J = 8.25 Hz, 1 H), 7.78 (d, J = 7.34 Hz, 1 H), 8.07 (s, 1 H). 13C-NMR (151 MHz, CDCl3) δ ppm 18.83, 47.27, 48.42, 56.15, 58.07, 62.80 (s), 102.92, 107.25, 123.45, 126.45, 127.75, 130.58, 131.85, 137.24, 150.61, 152.76, 197.03. MS (ESI m/z) 480.4 [M]+. Anal. Calcd. For: C20H21BrN2O5S: C, 49.90; H, 4.40. Found: C, 50.10; H, 4.51.
3.6. General Method for Synthesis of Benzo[4,5]isothiazolo[2,3-a]pyrazin-1-yl)(phenyl) Methanones (11–19)
To a stirred solution of chalcone dibromo sulphonyl chloride (1 g) in ethanol (25 mL), was added 2-methyl-1,2-diaminopropane (2 mole equivalent). The reaction mixture was warmed in a water bath for 10–15 min until all the solids had dissolved. The reaction mixture was allowed to cool to room temperature and the resulting solid was filtered and air dried. The product was purified by recrystallization from ethanol.
(8,9-Dimethoxy-3,3-dimethyl-6,6-dioxido-2,3,4,10b-tetrahydro-1H-benzo[4,5]isothiazolo [2,3-a]pyrazin-1-yl)(phenyl)methanone (11). White needle shaped crystals; yield 34%; m.p. 163–164 °C. IR (KBr ν cm−1): 3420 (NH), 1676 (C=O), 1277, 1141 (SO2). 1H-NMR (600 MHz, CDCl3) δ ppm 1.24 (s, 3 H), 1.41 (s, 3 H), 2.96 (d, J = 12.84 Hz, 1 H), 3.46 (s, 3 H), 3.89 (s, 3 H), 3.66 (d, J = 12.84 Hz, 1 H), 3.78 (m, 1 H), 4.51 (m, 1 H), 4.59 (m, 1 H), 6.38 (s, 1 H), 7.22 (s, 1 H), 7.48 (m, 2 H), 7.61 (m, 1 H), 7.98 (d, J = 7.34 Hz, 2 H). 13C-NMR (151 MHz, CDCl3) δ ppm 16.98, 18.39, 33.43, 56.43, 56.59, 58.72, 71.70, 102.94, 104.95, 124.29, 125.66, 126.34, 127.99, 128.80, 129.20, 130.85, 131.49, 131.89, 131.97, 132.10, 135.72, 147.20, 153.35, 165.30, 192.55. MS (ESI m/z) 416.3 [M]+. Anal. Calcd. For: C21H24N2O5S: C, 60.56; H, 5.81. Found: C, 60.60; H, 5.82.
(8,9-Dimethoxy-3,3-dimethyl-6,6-dioxido-2,3,4,10b-tetrahydro-1H-benzo[4,5]isothiazolo[2,3-a]pyrazin-1-yl)(p-tolyl)methanone (12). Light yellow crystals; yield 64%; m.p. 205–206 °C. IR (KBr ν cm−1): 3312 (NH), 1660 (C=O), 1275, 1177 (SO2). 1H-NMR (600 MHz, CDCl3) δ ppm 1.22 (s, 3 H), 1.39 (s, 3 H), 2.40 (s, 3 H), 2.94 (d, J = 12.84 Hz, 1 H), 3.45 (s, 3 H), 3.64 (d, J = 12.84 Hz, 1 H), 3.89 (s, 3 H), 4.47 (d, J = 9.17 Hz, 1 H), 4.55 (m, 1 H), 6.36 (s, 1 H), 7.21 (s, 1 H), 7.25 (d, J = 8.25 Hz, 2 H), 7.86 (d, J = 8.25 Hz, 2 H). 13C-NMR (151 MHz, CDCl3) δ ppm 20.23, 23.07, 28.32, 49.66, 49.95, 56.08, 56.44, 58.34, 58.52, 102.89, 106.71, 123.49, 126.89, 127.73, 130.64, 132.03, 137.24, 137.33, 150.66, 152.85, 197.35. MS (ESI m/z) 430.3 [M]+. Anal. Calcd. For: C22H26N2O5S: C, 61.38; H, 6.09. Found: C, 61.30; H, 6.10.
(8,9-Dimethoxy-3,3-dimethyl-6,6-dioxido-2,3,4,10b-tetrahydro-1H-benzo[4,5]isothiazolo[2,3-a]pyrazin-1-yl)(4-fluorophenyl)methanone (13). White crystals; yield 12%; m.p. 175–176 °C. IR (KBr ν cm−1): 3438 (NH), 1677 (C=O), 1289, 1180 (SO2). 1H-NMR (600 MHz, CDCl3) δ ppm 1.24 (s, 3 H), 1.41 (s, 3 H), 3.54 (s, 3 H), 3.66 (d, J = 13.75 Hz, 1 H), 3.90 (s, 3 H), 4.44 (d, J = 10.09 Hz, 1 H), 4.61 (d, J = 9.17 Hz, 1 H), 6.42 (s, 1 H), 7.15 (t, J = 8.71 Hz, 2 H), 7.23 (s, 1 H), 8.03 (m, 2 H). 13C-NMR (151 MHz, CDCl3) δ ppm 23.06, 28.27, 49.89, 55.98, 56.44, 58.13, 58.43, 102.87, 106.74, 116.33, 116.47, 126.89, 132.00, 150.66, 152.78, 165.72, 167.43, 204.13. MS (ESI m/z) 434.3 [M]+. Anal. Calcd. For: C21H23FN2O5S: C, 58.05; H, 5.34. Found: C, 58.10; H, 5.30.
(8,9-Dimethoxy-3,3-dimethyl-6,6-dioxido-2,3,4,10b-tetrahydro-1H-benzo[4,5]isothiazolo[2,3-a]pyrazin-1-yl)(4-Chlorophenyl)methanone (14). Light yellow crystals; yield 39%; m.p. 180–181 °C. IR (KBr ν cm−1): 3442 (NH), 1665 (C=O), 1290, 1182 (SO2). 1H-NMR (600 MHz, CDCl3) δ ppm 1.22 (s, 3 H), 1.39 (s, 3 H), 2.93 (d, J = 12.84 Hz, 1 H), 3.55 (s, 3 H), 3.66 (d, J = 13.75 Hz, 1 H), 3.89 (s, 3 H), 4.41 (d, J = 9.17 Hz, 1 H), 4.59 (d, J = 9.17 Hz, 1 H), 6.41 (s, 1 H), 7.22 (s, 1 H), 7.44 (d, J = 9.17 Hz, 2 H), 7.93 (m, 2 H). 13C-NMR (151 MHz, CDCl3) δ ppm 23.13, 28.36, 49.53, 49.97, 56.00, 56.43, 58.32, 58.41, 76.89, 77.10, 77.31, 102.86, 106.73, 126.90, 127.06, 129.47, 130.56, 133.87, 141.17, 150.62, 152.78, 197.41. MS (ESI m/z) 450.2 [M]+. Anal. Calcd. For: C21H23ClN2O5S: C, 55.94; H, 5.14. Found: C, 55.90; H, 5.20.
(8,9-Dimethoxy-3,3-dimethyl-6,6-dioxido-2,3,4,10b-tetrahydro-1H-benzo[4,5]isothiazolo[2,3-a]pyrazin-1-yl)(4-bromophenyl)methanone (15). Light yellow crystals; yield 45%; m.p. 195–196 °C. IR (KBr ν cm−1): 3447 (NH), 1663 (C=O), 1290, 1182 (SO2). 1H-NMR (600 MHz, CDCl3) δ ppm 1.22 (s, 3 H), 1.39 (s, 3 H), 2.93 (d, J = 13.76 Hz, 1 H), 3.55 (s, 3 H), 3.66 (d, J = 13.75 Hz, 1 H), 3.90 (s, 3 H), 4.40 (d, J = 9.17 Hz, 1 H), 4.59 (d, J = 9.17 Hz, 1 H), 6.41 (s, 1 H), 7.22 (s, 1 H), 7.61 (d, J = 9.17 Hz, 2 H), 7.84 (m, 2 H). 13C-NMR (152 MHz, CDCl3) δ ppm 23.13, 28.35, 49.55, 49.97, 56.00, 56.44, 58.31, 58.40, 76.88, 77.10, 77.31, 102.86, 106.73, 126.89, 127.04, 129.98, 130.61, 132.48, 134.26, 150.62, 152.79, 197.63. MS (ESI m/z) 494.1 [M]+. Anal. Calcd. For: C21H23BrN2O5S: C, 50.92; H, 4.68. Found: C, 50.95; H, 4.67.
(8,9-Dimethoxy-3,3-dimethyl-6,6-dioxido-2,3,4,10b-tetrahydro-1H-benzo[4,5]isothiazolo[2,3-a]pyrazin-1-yl)(3-chlorophenyl)methanone (16). Light yellow crystals; yield 54%; m.p. 179–180 °C. IR (KBr ν cm−1): 3449 (NH), 1681 (C=O), 1275, 1190 (SO2). 1H-NMR (600 MHz, CDCl3) δ ppm 1.24 (s, 3 H), 1.42 (s, 3 H), 2.95 (d, J = 13.75 Hz, 1 H), 3.58 (s, 3 H), 3.67 (d, J = 12.84 Hz, 1 H), 3.90 (s, 3 H), 4.41 (d, J = 9.17 Hz, 1 H), 4.61 (d, J = 9.17 Hz, 1 H), 6.42 (s, 1 H), 7.23 (s, 1 H), 7.42 (t, J = 7.79 Hz, 1 H), 7.58 (m, 1 H), 7.84 (d, J = 8.25 Hz, 1 H), 7.99 (t, J = 1.83 Hz, 1 H). 13C-NMR (151 MHz, CDCl3) δ ppm 23.05, 28.26, 49.91, 56.06, 56.44, 58.34, 58.48, 102.89, 106.70, 126.89, 127.31, 129.06, 130.43, 134.36, 137.14, 150.66, 152.84. MS (ESI m/z) 450.2 [M]+. Anal. Calcd. For: C21H23ClN2O5S: C, 55.94; H, 5.14. Found: C, 56.10; H, 5.23.
(8,9-Dimethoxy-3,3-dimethyl-6,6-dioxido-2,3,4,10b-tetrahydro-1H-benzo[4,5]isothiazolo[2,3-a]pyrazin-1-yl)(3-bromophenyl)methanone (17). Light yellow crystals; yield 80%; m.p. 175–176 °C. IR (KBr ν cm−1): 3447 (NH), 1681 (C=O), 1265, 1190 (SO2). 1H-NMR (600 MHz, CDCl3) δ ppm 1.24 (s, 3 H), 1.42 (s, 3 H), 2.93 (m, 1 H), 3.70 (m, 1 H), 3.87 (s, 3 H), 3.90 (s, 3 H), 4.41 (d, J = 9.17 Hz, 1 H), 4.61 (d, J = 9.17 Hz, 1 H), 6.42 (s, 1 H), 7.23 (s, 1 H), 7.35 (t, J = 7.79 Hz, 1 H), 7.73 (d, J = 9.17 Hz, 1 H), 7.87 (d, J = 7.34 Hz, 1 H), 8.14 (t, J = 1.83 Hz, 1 H). 13C-NMR (151 MHz, CDCl3) δ ppm 21.84, 23.27, 28.42, 49.30, 50.01, 55.87, 56.40, 57.94, 58.89, 102.75, 106.84, 126.81, 127.22, 129.28, 129.83, 133.25, 145.75, 150.46, 152.64, 198.16. MS (ESI m/z) 495.9 [M]+. Anal. Calcd. For: C21H23BrN2O5S: C, 50.92; H, 4.68. Found: C, 50.90; H, 4.60.
(8,9-Dimethoxy-3,3-dimethyl-6,6-dioxido-2,3,4,10b-tetrahydro-1H-benzo[4,5]isothiazolo[2,3-a]pyrazin-1-yl)(2-chlorophenyl)methanone (18). White crystals; yield 64%; m.p. 191–192 °C. IR (KBr ν cm−1): 3448 (NH), 1720 (C=O), 1297, 1179 (SO2). 1H-NMR (600 MHz, CDCl3) δ ppm 1.43 (s, 3 H), 1.57 (s, 3 H), 2.96 (dd, J = 16.05, 11.46 Hz, 1 H), 3.20 (d, J = 13.75 Hz, 1 H), 3.39 (m, 1 H), 3.67 (d, J = 14.67 Hz, 1 H), 3.92 (s, 3 H), 3.94 (s, 3 H), 4.63 (d, J = 11.00 Hz, 1 H), 6.75 (s, 1 H), 7.23 (s, 1 H), 7.32 (d, J = 2.75 Hz, 3 H), 7.40 (m, 1 H). 13C-NMR (151 MHz, CDCl3) δ ppm 23.26, 31.34, 42.19, 50.76, 56.54, 58.60, 60.05, 102.91, 104.78, 125.99, 127.55, 129.32, 129.70, 129.87, 130.01, 130.95, 143.44, 150.77, 153.75, 164.95. MS (ESI m/z) 450.2 [M]+. Anal. Calcd. For: C21H23ClN2O5S: C, 55.94; H, 5.14. Found: C, 55.92; H, 5.17.
(8,9-Dimethoxy-3,3-dimethyl-6,6-dioxido-2,3,4,10b-tetrahydro-1H-benzo[4,5]isothiazolo[2,3-a]pyrazin-1-yl)(2-bromophenyl)methanone (19). White crystals; yield 29%; m.p. 201–202 °C. IR (KBr ν cm−1): 3467 (NH), 1715 (C=O), 1297, 1179 (SO2). 1H-NMR (600 MHz, CDCl3) δ ppm 1.43 (s, 3 H), 1.57 (s, 3 H), 2.95 (dd, J = 15.59, 11.92 Hz, 1 H), 3.21 (d, J = 13.75 Hz, 1 H), 3.36 (m, 1 H), 3.67 (d, J = 14.67 Hz, 1 H), 3.92 (s, 3 H), 3.93 (s, 3 H), 4.72 (d, J = 11.00 Hz, 1 H), 6.78 (s, 1 H), 7.23 (s, 1 H), 7.25 (m, 1 H), 7.29 (m, 1 H), 7.36 (m, 1 H), 7.59 (d, J = 8.25 Hz, 1 H). 13C-NMR (151 MHz, CDCl3) δ ppm 23.49, 31.11, 42.25, 50.81, 56.53, 58.88, 60.09, 76.88, 77.09, 77.30, 102.91, 104.76, 120.07, 125.96, 128.09, 129.33, 129.70, 130.09, 132.97, 145.30, 150.77, 153.77, 165.54. MS (ESI m/z) 498.6 [M]+. Anal. Calcd. For: C21H23BrN2O5S: C, 50.92; H, 4.68. Found: C, 50.85; H, 4.75.
3.7. Antimicrobial Activity
The benzo[4,5]isothiazolo[2,3-
a]pyrazine-6,6-dioxide derivatives
2–
19 were screened against four bacteria,
B. subtilis ATCC 6633,
E. coli ATCC 25922,
P. vulgaris ATCC 13315 (ATCC 29905) and
S. aureus ATCC 6538. The broth micro dilution method (with a slight modification as described by the Clinical and Laboratory Standards Institute) using 96-well micro-titre plates was used to test the antibacterial activity of the compounds
2–
19 [
29]. The concentration of the compounds was tested in a range of 6.67 mg·mL
−1 down to 0.052 mg·mL
−1. Dilutions were carried out so that each well had 50 μL of Mueller–Hinton broth with a particular concentration of the compound. An equal volume of bacterial culture adjusted to 0.5 McFarland turbidity standard and further diluted to 1:20 was added to the wells and incubated at 37 °C for 16–18 h. Plates were then examined for turbidity as an indicator of growth, and the absorbance was taken using an ELISA reader at 590 nm [
30]. The Minimum Inhibitiry Concentration (MIC) of the test compounds were further confirmed by adding a concentration of 5 mg/mL MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] was prepared in Phosphate Buffered Saline (PBS) (pH 7.2). Twenty μL of MTT solution was added to each single well and the 96-well micro-titer plates were incubated for 30 min at 30 °C. The wells with lowest concentration of the compound where there was no formazan were confirmed as the MIC of the test compounds.
Streptomycin was used as a reference drug throughout all the antibacterial testing.