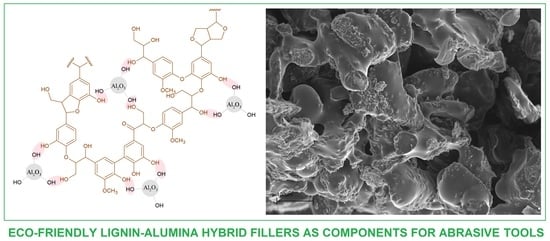
Characteristics of Multifunctional, Eco-Friendly Lignin-Al2O3 Hybrid Fillers and Their Influence on the Properties of Composites for Abrasive Tools
Abstract
:1. Introduction
2. Results
2.1. Dispersive-Morphological Properties of Lignin-Al2O3 Hybrids
2.2. Fourier Transform Infrared Spectroscopy
2.3. Thermogravimetric Analysis–Mass Spectrometry
2.4. Inverse Gas Chromatography
2.5. Rheological Studies
2.6. Dynamic-Mechanical Properties
2.7. Scanning Electron Microscopy Analysis of Composites
2.8. Assessment of Emission of Phenol and Formaldehyde by Means of HS-GC Analysis
3. Materials and Methods
3.1. Preparation of Novel Lignin-Al2O3 Hybrid Filler
3.2. Preparation of Abrasive Composites with Lignin-Al2O3 Hybrids
3.3. Physicochemical and Dispersive-Morphological Characteristics of Lignin-Alumina Hybrids
3.3.1. Particle Size Distribution
3.3.2. Scanning Electron Microscopy
3.3.3. Fourier Transform Infrared Spectroscopy
3.3.4. Thermogravimetric Analysis—Mass Spectrometry
3.3.5. Inverse Gas Chromatography
3.4. Rheological Studies
3.5. Dynamic-Mechanical Properties
3.6. Headspace Gas Chromatography
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Voelkel, A.; Strzemiecka, B. Characterization of fillers used in abrasive articles by means of inverse gas chromatography and principal component analysis. Int. J. Adhes. Adhes. 2007, 27, 188–194. [Google Scholar] [CrossRef]
- Voelkel, A.; Strzemiecka, B.; Jesionowski, T. The examination of the degree of coverage of the fused alumina abrasive by resol wetting agent by Inverse GC. Chroma 2009, 70, 1393–1397. [Google Scholar] [CrossRef]
- Strzemiecka, B.; Voelkel, A.; Hinz, M.; Rogozik, M. Application of inverse gas chromatography in physicochemical characterization of phenolic resin adhesives. J. Chromatogr. A 2014, 1368, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Laza, J.M.; Alonso, J.; Vilas, J.L.; Rodríguez, M.; León, L.M.; Gondra, K.; Ballestero, J. Influence of fillers on the properties of a phenolic resin cured in acidic medium. J. Appl. Polym. Sci. 2008, 108, 387–392. [Google Scholar] [CrossRef]
- Strzemiecka, B.; Voelkel, A.; Chmielewska, D.; Sterzyński, T. Influence of different fillers on phenolic resin abrasive composites. Comparison of inverse gas chromatographic and dynamic mechanical-thermal analysis characteristics. Int. J. Adhes. Adhes. 2014, 51, 81–86. [Google Scholar] [CrossRef]
- Dang, A.; Ojha, S.; Hui, C.M.; Mahoney, C.; Matyjaszewski, K.; Bockstaller, M.R. High-transparency polymer nanocomposites enabled by polymer-graft modification of particle fillers. Langmuir 2014, 30, 14434–14442. [Google Scholar] [CrossRef] [PubMed]
- Imre, B.; Pukánszky, B. From natural resources to functional polymeric biomaterials. Eur. Polym. J. 2015, 68, 481–487. [Google Scholar] [CrossRef]
- Cataldi, A.; Deflorian, F.; Pegoretti, A. Microcrystalline cellulose filled composites for wooden artwork consolidation: Application and physic-mechanical characterization. Mater. Des. 2015, 83, 611–619. [Google Scholar] [CrossRef]
- Cataldi, A.; Dorigato, A.; Deflorian, F.; Pegoretti, A. Thermo-mechanical properties of innovative microcrystalline cellulose filled composites for art protection and restoration. J. Mater. Sci. 2014, 49, 2035–2044. [Google Scholar] [CrossRef]
- Bozsódi, B.; Romhányi, V.; Pataki, P.; Kun, D.; Renner, K.; Pukánszky, B. Modification of interactions in polypropylene/lignosulfonate blends. Mater. Des. 2016, 103, 32–39. [Google Scholar] [CrossRef]
- Milczarek, G.; Inganäs, O. Renewable cathode materials from biopolymer/conju-gated polymer interpenetrating networks. Science 2012, 335, 1468–1471. [Google Scholar] [CrossRef] [PubMed]
- Milczarek, G. Lignosulfonate-modified electrodes: Electrochemical properties and electrocatalysis of NADH oxidation. Langmuir 2009, 25, 10345–10353. [Google Scholar] [CrossRef] [PubMed]
- Jesionowski, T.; Klapiszewski, Ł.; Milczarek, G. Kraft lignin and silica as precursors of advanced composite materials and electroactive blends. J. Mater. Sci. 2014, 49, 1376–1385. [Google Scholar] [CrossRef]
- Jesionowski, T.; Klapiszewski, Ł.; Milczarek, G. Structural and electrochemical properties of multifunctional silica/lignin materials. Mater. Chem. Phys. 2014, 147, 1049–1057. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Hatakeyama, T. Lignin structure, properties, and application. Adv. Polym. Sci. 2010, 232, 1–63. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, M.R. Progress in green polymer composites from lignin for multifunctional applications: A review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Gupta, R.K. Review: Raw natural fiber-based polymer composites. Int. J. Polym. Anal. Charact. 2014, 19, 256–271. [Google Scholar] [CrossRef]
- El-Zawawy, W.K.; Ibrahim, M.M.; Belgacem, M.N.; Dufresne, A. Characterization of the effects of lignin and lignin complex particles as filler on a polystyrene film. Mater. Chem. Phys. 2011, 131, 348–357. [Google Scholar] [CrossRef]
- Faludi, G.; Hári, J.; Renner, K.; Móczó, J.; Pukánszky, B. Fiber association and network formation in PLA/lignocellulosic fiber composites. Compos. Sci. Technol. 2013, 77, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Faludi, G.; Dora, G.; Renner, K.; Móczó, J.; Pukánszky, B. Biocomposite from polylactic acid and lignocellulosic fibers: Structure-property correlations. Carbohydr. Polym. 2013, 92, 1767–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klapiszewski, Ł.; Pawlak, F.; Tomaszewska, J.; Jesionowski, T. Preparation and characterization of novel PVC/silica-lignin composites. Polymers 2015, 7, 1767–1788. [Google Scholar] [CrossRef]
- Bula, K.; Klapiszewski, Ł.; Jesionowski, T. A novel functional silica/lignin hybrid material as a potential bio-based polypropylene filler. Polym. Compos. 2015, 36, 913–922. [Google Scholar] [CrossRef]
- Kharade, A.Y.; Kale, D.D. Effect of lignin on phenolic novolak resins and moulding powder. Eur. Polym. J. 1998, 34, 201–205. [Google Scholar] [CrossRef]
- Hattali, S.; Benaboura, A.; Dumarçay, S.; Gérardin, P. Evaluation of alfa grass soda lignin as a filler for novolak molding powder. J. Appl. Polym. Sci. 2005, 97, 1065–1068. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, S.; Shan, X. Adsorption of metal ions on lignin. J. Hazard. Mater. 2008, 151, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Klapiszewski, Ł.; Bartczak, P.; Wysokowski, M.; Jankowska, M.; Kabat, K.; Jesionowski, T. Silica conjugated with kraft lignin and its use as a novel ‘green’ sorbent for hazardous metal ions removal. Chem. Eng. J. 2015, 260, 684–693. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Z.; Kong, Y.; Song, Q.; Wang, K. Heavy metal ions retention by bi-functionalized lignin: Synthesis, applications, and adsorption mechanisms. J. Ind. Eng. Chem. 2014, 20, 4429–4436. [Google Scholar] [CrossRef]
- Qu, Y.; Tian, Y.; Zou, B.; Zhang, J.; Zheng, Y.; Wang, L.; Li, Y.; Rong, C.; Wang, Z. A novel mesoporous lignin-silica hybrid from rice husk produced by a sol-gel method. Bioresour. Technol. 2010, 101, 8402–8405. [Google Scholar] [CrossRef] [PubMed]
- Klapiszewski, Ł.; Nowacka, M.; Milczarek, G.; Jesionowski, T. Physicochemical and electrokinetic properties of silica-lignin biocomposites. Carbohydr. Polym. 2013, 94, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Klapiszewski, Ł.; Rzemieniecki, T.; Krawczyk, M.; Malina, D.; Norman, M.; Zdarta, J.; Majchrzak, I.; Dobrowolska, A.; Czaczyk, K.; Jesionowski, T. Kraft lignin/silica-AgNPs as a functional material with antibacterial activity. Coll. Surf. B 2015, 134, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh-Tabrizi, S.A.; Taheri-Nassaj, E. Economical synthesis of Al2O3 nanopowder using a precipitation method. Mater. Lett. 2009, 63, 2274–2276. [Google Scholar] [CrossRef]
- Costa, T.M.H.; Gallas, M.R.; Benvenutti, E.V.; da Jornada, J.A.H. Study of nanocrystalline γ-Al2O3 produced by high-pressure compaction. J. Phys. Chem. B 1999, 103, 4278–4284. [Google Scholar] [CrossRef]
- Naskar, M.K. Hydrothermal synthesis of petal-like alumina flakes. J. Am. Ceram. Soc. 2009, 92, 2392–2395. [Google Scholar] [CrossRef]
- Rahman, M.A.; de Santis, D.; Spagnoli, G.; Ramorino, G.; Penco, M.; Phuong, V.T.; Lazzeri, A. Biocomposites based on lignin and plasticized poly(l-lactic acid). J. Appl. Polym. Sci. 2013, 129, 202–214. [Google Scholar] [CrossRef]
- Mancera, C.; Ferrando, F.; Salvadó, J.; El Mansouri, N.E. Kraft lignin behavior during reaction in an alkaline medium. Biomass Bioenerg. 2011, 35, 2072–2079. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal degradation of lignin—A review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Jakab, E.; Faix, O.; Till, F. Thermal decomposition of milled wood lignins studied by thermogravimetry/mass spectrometry. J. Anal. Appl. Pyrol. 1997, 40–41, 171–186. [Google Scholar] [CrossRef]
- Matsushita, Y.; Wada, S.; Fukushima, K.; Yasuda, S. Surface characteristics of phenol-formaldehyde-lignin resin determined by contact angle measurement and inverse gas chromatography. Ind. Crop. Prod. 2006, 23, 115–121. [Google Scholar] [CrossRef]
- Faulstich de Paiva, J.M.; Frollini, E. Unmodified and modified surface sisal fibers as reinforcement of phenolic and lignophenolic matrices composites: Thermal analyses of fibers and composites. Macromol. Mater. Eng. 2006, 291, 405–417. [Google Scholar] [CrossRef]
- Menard, K.P. Dynamic Mechanical Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; ISBN 9781420053128. [Google Scholar]
- Brostow, W.; Hagg Lobland, H.E. Materials: Introduction and Applications; John Wiley & Sons: New York, NY, USA, 2017; ISBN 978-0-470-52379-7. [Google Scholar]
- Kalogeras, I.M.; Hagg Lobland, H.E. The nature of the glassy state: Structure and glass transitions. J. Mater. Educ. 2012, 34, 69–94. [Google Scholar]
- Kelley, S.; Rials, T.G.; Glasser, W.G. Relaxation behaviour of the amorphous components of wood. J. Mater. Sci. 1987, 22, 617–624. [Google Scholar] [CrossRef]
- Wang, J.; Laborie, M.P.G.; Wolcott, M.P. Kinetic analysis of phenol-formaldehyde bonded wood joints with dynamical mechanical analysis. Thermochim. Acta 2009, 491, 58–62. [Google Scholar] [CrossRef]
- Guigo, N.; Mija, A.; Vincent, L.; Sbirrazzuoli, N. Eco-friendly composite resins based on renewable biomass resources: Polyfurfuryl alcohol/lignin thermosets. Eur. Polym. J. 2010, 46, 1016–1023. [Google Scholar] [CrossRef]
- Gardziella, A.; Pilato, L.A.; Knop, A. Phenolic Resins-Chemistry, Applications, Standardization, Safety and Ecology; Springer: Berlin, Germany, 2000; ISBN 3540655174. [Google Scholar]
- Schultz, J.; Lavielle, L.; Martin, C. Proprietes de surface des fibres de carbone dèterminèes par chromatographie gazeuse inverse. J. Chim. Phys. 1987, 84, 231–237. [Google Scholar] [CrossRef]
- Oss, C.; Good, R.; Chaudhury, M. Additive and nonadditive surface tension components and the interpretation of contact angles. Langmuir 1988, 4, 884–891. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |











| Sample | Dispersive Properties | ||||
|---|---|---|---|---|---|
| Particle Size Distribution from Zetasizer Nano ZS (nm) | Particle Diameter from Mastersizer 2000 (µm) | ||||
| d(0.1) * | d(0.5) ** | d(0.9) *** | D(4.3) **** | ||
| Al2O3 | 142–955 | 2.5 | 3.7 | 5.5 | 3.7 |
| Lignin-Al2O3 (8:1, wt/wt) | 531–1106 | 2.1 | 3.6 | 5.3 | 3.3 |
| Lignin-Al2O3 (8:2, wt/wt) | 396–825 | 2.5 | 3.7 | 5.2 | 3.4 |
| Lignin-Al2O3 (8:4, wt/wt) | 295–955 | 2.4 | 3.5 | 5.2 | 3.2 |
| Lignin-Al2O3 (8:6, wt/wt) | 142–825 | 2.4 | 3.6 | 5.3 | 3.4 |
| Lignin | Alumina | Lignin-Al2O3 Hybrid | Vibrational Assignment |
|---|---|---|---|
| - | 3635, 3543 and 3473 | overshadowed | Al–OH stretching |
| 3432 | 3145 | 3430 | O–H stretching, absorbed water |
| 2935, 2877 | - | 2937, 2879 | CHx stretching |
| 1648 | - | 1646 | C=O stretching |
| 1618 | 1620 | 1619 | O–H bending of water |
| 1602 not visible | - | 1602 not visible | C–C, C=C (aromatic skeleton), stretching |
| 1508 | - | 1508 | |
| 1471 | - | 1470 | C–H (CH3 + CH2), bending |
| 1419 | - | 1418 | C–C, C=C (aromatic skeleton), stretching |
| - | 1390 | 1389 | Al–O as Si cage (TO4) |
| 1271 | - | 1271 | C–O (guaiacyl unit) stretching |
| 1226 | - | 1226 | C–OH (phenolic OH) stretching |
| 1139 | - | 1140 | Aromatic C–H (guaiacyl unit), stretching |
| 1080 | - | 1077 | C–O stretching |
| 1045 | - | 1039 | C–OH + C–O–C (aliphatic OH + ether) stretching |
| - | 1035 | 1039 | Al–OH symmetric bending |
| - | 970, 893 | 969, 893 | –OH deformation linked to Al3− |
| 856, 751 | - | 858, 751 | Aromatic C–H (guaiacyl unit), bending |
| - | 788, 750, 693, 564 and 512 | 788, 751, 695, 565 and 512 | Al–O in which aluminum ions are in both tetrahedral and octahedral sites |
| 534 | - | 534 | CHx bending |
| Sample Composition | Tonset from DSC (°C) | |||
|---|---|---|---|---|
| Lignin | Lignin-Al2O3 Hybrid Fillers (wt/wt) | |||
| 8:1 | 8:2 | 8:4 | 8:6 | |
| 34 | 32 | 27 | 28 | 28 |
| 324 | 330 | 329 | 328 | 327 |
| 650 | 645 | 678 | 646 | 640 |
| Speed of Decomposition from DTG (%/min) | ||||
| Lignin | Lignin-Al2O3 Hybrid Fillers | |||
| 8:1 | 8:2 | 8:4 | 8:6 | |
| 0.85 | 0.72 | 0.69 | 0.50 | 0.58 |
| 2.14 | 1.98 | 1.76 | 1.43 | 1.23 |
| 0.65 | 0.72 | 0.70 | 0.71 | 0.20 |
| Sample Mass Loss (%), Where Temperature Ranges Are, a: RT-160 °C, b: 160–550 °C, c: 550–1000 °C | ||||
| 6.8 a | 5.9 a | 5.6 a | 4.4 a | 4.1 a |
| 33.8 b | 31.0 b | 28.1 b | 23.6 b | 20.4 b |
| 20.2 c | 13.2 c | 13.0 c | 12.2 c | 9.2 c |
| Sample | (mJ/m2) | (mJ/m2) | (mJ/m2) | KA (-) | KD (-) | KA/KD (-) |
|---|---|---|---|---|---|---|
| Al2O3 | 37.2 ± 0.5 | 29.0 ± 0.1 | 177.2 ± 5.0 | 0.100 ± 0.010 | 0.260 ± 0.003 | 0.385 |
| Lignin | 35.2 ± 0.6 | 15.2 ± 0.2 | 46.4 ± 1.0 | 0.112 ± 0.005 | 0.161 ± 0.002 | 0.702 |
| Lignin-Al2O3 (8:1, wt/wt) | 33.2 ± 0.4 | 11.5 ± 0.1 | 38.2 ± 1.1 | 0.071 ± 0.001 | 0.140 ± 0.004 | 0.507 |
| Lignin-Al2O3 (8:2, wt/wt) | 36.4 ± 0.3 | 9.8 ± 0.2 | 35.8 ± 0.8 | 0.067 ± 0.001 | 0.143 ± 0.007 | 0.469 |
| Lignin-Al2O3 (8:4, wt/wt) | 35.6 ± 0.1 | 10.5 ± 0.1 | 39.3 ± 0.5 | 0.068 ± 0.001 | 0.146 ± 0.003 | 0.466 |
| Lignin-Al2O3 (8:6, wt/wt) | 35.7 ± 0.1 | 27.1 ± 0.3 | 148.1 ± 3.0 | 0.101 ± 0.002 | 0.220 ± 0.003 | 0.459 |
| Sample | Softening Point | Cross Over Point | ||
|---|---|---|---|---|
| Temperature (°C) | Viscosity (Pa·s) | Temperature (°C) | G′ = G″ (Pa) | |
| Al2O3 | 136.4 | 1.029 | 158 | 27,400 |
| Lignin | 136.1 | 49.68 | 157 | 898,000 |
| Lignin-Al2O3 (8:1, wt/wt) | 135.5 | 3950 | 158 | 430,000 |
| Lignin-Al2O3 (8:2, wt/wt) | 135.7 | 2913 | 160 | 533,000 |
| Lignin-Al2O3 (8:4, wt/wt) | 136.7 | 1294 | 158 | 680,000 |
| Lignin-Al2O3 (8:6, wt/wt) | 135.6 | 633 | 160 | 326,000 |
| Sample | G′ 25 °C (MPa) | G′ 50 °C (MPa) | G′ 300 °C (MPa) | Tan δmax | Tg (°C) |
|---|---|---|---|---|---|
| Reference sample | 2750 | 2680 | 1350 | 0.055 | 252 |
| Lignin-Al2O3 (8:1, wt/wt) | 1690 | 1640 | 776 | 0.076 | 244 |
| Lignin-Al2O3 (8:2, wt/wt) | 1150 | 1110 | 530 | 0.067 | 250 |
| Lignin-Al2O3 (8:4, wt/wt) | 1520 | 1470 | 691 | 0.068 | 247 |
| Lignin-Al2O3 (8:6, wt/wt) | 1470 | 1420 | 652 | 0.071 | 244 |
| Sample | Peak Area, Speak (µV·s) * |
|---|---|
| Novolac | 1.21 × 106 ± 0.11 × 106 |
| Resol | 1.90 × 106 ± 0.28 × 106 |
| Kraft lignin | 2.52 × 106 ± 0.09 × 106 |
| Lignin-Al2O3 (8:4, wt/wt) | 2.30 × 106 ± 0.08 × 106 |
| Resol + novolac + Kraft lignin | 2.73 × 106 ± 0.18 × 106 |
| Resol + novolac + lignin-Al2O3 (8:4, wt/wt) | 2.24 × 106 ± 0.18 × 106 |
| Resol + novolac + zeolite micro 20 | 2.05 × 106 ± 0.20 × 106 |
| Sample | The Peak Area, Speak (µV·s) * |
|---|---|
| Novolac | 0.32 × 106 ± 0.05 × 106 |
| Resol | 10.53 × 106 ± 0.60 × 106 |
| Kraft lignin | 0.02 × 106 ± 0.00 × 106 |
| Lignin-Al2O3 (8:4, wt/wt) | 0.01 × 106 ± 0.00 × 106 |
| Resol + novolac + Kraft lignin | 5.49 × 106 ± 0.40 × 106 |
| Resol + novolac + lignin-Al2O3 (8:4, wt/wt) | 3.47 × 106 ± 0.31 × 106 |
| Resol + novolac + zeolite micro 20 | 4.86 × 106 ± 0.45 × 106 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klapiszewski, Ł.; Jamrozik, A.; Strzemiecka, B.; Koltsov, I.; Borek, B.; Matykiewicz, D.; Voelkel, A.; Jesionowski, T. Characteristics of Multifunctional, Eco-Friendly Lignin-Al2O3 Hybrid Fillers and Their Influence on the Properties of Composites for Abrasive Tools. Molecules 2017, 22, 1920. https://doi.org/10.3390/molecules22111920
Klapiszewski Ł, Jamrozik A, Strzemiecka B, Koltsov I, Borek B, Matykiewicz D, Voelkel A, Jesionowski T. Characteristics of Multifunctional, Eco-Friendly Lignin-Al2O3 Hybrid Fillers and Their Influence on the Properties of Composites for Abrasive Tools. Molecules. 2017; 22(11):1920. https://doi.org/10.3390/molecules22111920
Chicago/Turabian StyleKlapiszewski, Łukasz, Artur Jamrozik, Beata Strzemiecka, Iwona Koltsov, Bartłomiej Borek, Danuta Matykiewicz, Adam Voelkel, and Teofil Jesionowski. 2017. "Characteristics of Multifunctional, Eco-Friendly Lignin-Al2O3 Hybrid Fillers and Their Influence on the Properties of Composites for Abrasive Tools" Molecules 22, no. 11: 1920. https://doi.org/10.3390/molecules22111920
APA StyleKlapiszewski, Ł., Jamrozik, A., Strzemiecka, B., Koltsov, I., Borek, B., Matykiewicz, D., Voelkel, A., & Jesionowski, T. (2017). Characteristics of Multifunctional, Eco-Friendly Lignin-Al2O3 Hybrid Fillers and Their Influence on the Properties of Composites for Abrasive Tools. Molecules, 22(11), 1920. https://doi.org/10.3390/molecules22111920