Chemical Characterization and Antioxidant Potential of Wild Ganoderma Species from Ghana
Abstract
:1. Introduction
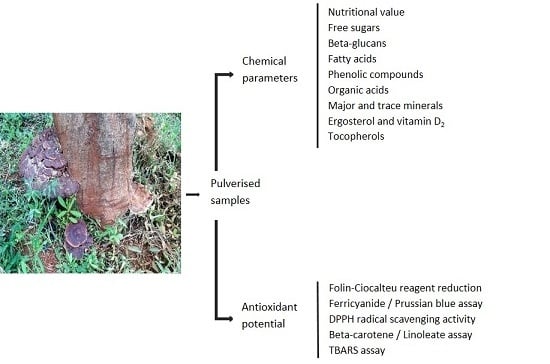
2. Results and Discussion
2.1. Nutritional, Free Sugars and Beta-Glucans Composition
2.2. Fatty Acids
2.3. Phenolic Compounds and Organic Acids
2.4. Major and Trace Minerals
2.5. Ergosterol, Vitamin D2 and Tocopherols
2.6. Antioxidant Activity
3. Materials and Methods
3.1. Mushroom Species
3.2. Standards and Reagents
3.3. Chemical Parameters
3.3.1. Nutritional and Energetic Value
3.3.2. Free Sugars
3.3.3. Beta-Glucans
3.3.4. Fatty Acids
3.3.5. Phenolic Compounds
3.3.6. Organic Acids
3.3.7. Major and Trace Minerals Composition
3.3.8. Ergosterol and Vitamin D2
3.3.9. Tocopherols
3.4. Antioxidant Activity
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Otto, E.C.; Blanchette, R.A.; Held, B.W.; Barnes, C.W. Fungal Planet 395 Ganoderma wiiroense. Pers. Mol. Phylogeny Evol. Fungi 2015, 35, 316–317. [Google Scholar]
- Otto, E.C.; Blanchette, R.A.; Held, B.W.; Barnes, C.W.; Obodai, M. Fungal planet 449 Ganoderma mbrekobenum. Pers. Mol. Phylogeny Evol. Fungi 2016, 36, 416–417. [Google Scholar]
- Obodai, M.; Blanchette, R.A.; Barnes, C.W.; Otto, E.C.; Narh Mensah, D.L.; Dzomeku, M.; Prempeh, J.; Takli, R.K. Identification of Species within the Ganoderma lucidum Complex in Ghana; CSIR-FRI/RE/OM/2016/006; CSIR—Food Research Institute: Accra, Ghana, 2016. [Google Scholar]
- Ferreira, I.C.F.R.; Heleno, S.A.; Reis, F.S.; Stojkovic, D.; Queiroz, M.J.R.P.; Vasconcelos, M.H.; Sokovic, M. Chemical features of Ganoderma polysaccharides with antioxidant, antitumor and antimicrobial activities. Phytochemistry 2015, 114, 38–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paterson, R.R.M. Ganoderma—A therapeutic fungal biofactory. Phytochemistry 2006, 67, 1985–2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borchers, A.T.; Keen, C.L.; Gershwin, M.E. Mushrooms, tumors, and immunity: An update. Exp. Biol. Med. 2014, 229, 393–406. [Google Scholar] [CrossRef]
- Valverde, M.E.; Hernández-Perez, T.; Paredes-López, O. Edible mushrooms: Improving human health and promoting quality life. Int. J. Microbiol. 2013, 2015, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Seviour, R. Medicinal importance of fungal β-(1→3), (1→6)-glucans. Mycol. Res. 2007, 111, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Mau, J.L.; Tsai, S.Y.; Tseng, Y.H.; Huang, S.J. Antioxidant properties of methanolic extracts from Ganoderma tsugae. Food Chem. 2005, 93, 641–649. [Google Scholar] [CrossRef]
- Palacios, I.; Lozano, M.; Moro, C.; D’Arrigo, M.; Rostagno, M.A.; Martínez, J.A.; García-Lafuente, A.; Guillamón, E.; Villares, A. Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chem. 2011, 128, 674–678. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, B.K. Biomedical triterpenoids of Ganoderma lucidum (curt.:fr.) P. Karst. (Aphyllophoromycetideae). Int. J. Med. Mushrooms 1999, 1, 121–138. [Google Scholar] [CrossRef]
- Czub, J.; Baginski, M. Comparative molecular dynamics study of lipid membranes containing cholesterol and ergosterol. Biophys. J. 2006, 90, 2368–2382. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, K.; Greenspan, S.L.; Thomas, S.B.; Holick, M.F. Solar ultraviolet radiation and vitamin D: A historical perspective. Am. J. Public Health 2007, 97, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Raina, S.; Sodhi, H.S.; Sethi, S. Identification of Ergosterol in Mushrooms. In Proceedings of the 8th International Conference on Mushroom Biology and Mushroom Products (ICMBMP8), New Delhi, India, 19–22 November 2014; Singh, M., Ed.; ICAR-Directorate of Mushroom Research: New Delhi, India, 2014; pp. 247–251. [Google Scholar]
- Ogbe, A.O.; Ditse, U.; Echeonwu, I.; Ajodoh, K.; Atawodi, S.E.; Abdu, P.A. Potential of a Wild Medicinal Mushroom, Ganoderma sp., as Feed Supplement in Chicken Diet: Effect on Performance and Health of Pullets. Int. J. Poult. Sci. 2009, 11, 1052–1057. [Google Scholar] [CrossRef]
- Obodai, M.; Apetorgbor, M. An Ethnobotanical Study of Mushroom Germplasm and Its Domestication in the Bia Biosphere Reserve of Ghana; UNESCO through Environmental Protection Agency of Ghana: Accra, Ghana, 2001. [Google Scholar]
- Apetorgbor, M.M.; Apetorgbor, A.K.; Nutakor, E. Utilization and cultivation of edible mushrooms for rural livelihood in Southern Ghana. In Proceedings of the 17th Commonwealth Forestry Conference, Colombo, Sri Lanka, 28 February–5 March 2005; pp. 1–22.
- Osarenkhoe, O.O.; John, O.A.; Theophilus, D.A. Ethnomycological Conspectus of West African Mushrooms: An Awareness Document. Adv. Microbiol. 2014, 4, 39–54. [Google Scholar] [CrossRef]
- Obodai, M.; Dzomeku, M.; Mensah, D.N.; Takli, R. Mushroom Cultivation Technology in Ghana. Available online: http://wsmbmp.org/Bol14/1.html (accessed on 16 July 2016).
- Bano, Z.; Rajarathnam, S.; Steinkraus, K.H. Pleurotus mushrooms. Part II. Chemical composition, nutritional value, post-harvest physiology, preservation, and role as human food. Crit. Rev. Food Sci. Nutr. 1988, 27, 87–158. [Google Scholar] [CrossRef] [PubMed]
- Korley, K.J.N. Comparative Effect of Steam and Gamma Irradiation Sterilization of Sawdust Compost on the Yield, Nutrient and Shelf—Life of Pleurotus Ostreatus (Jacq. Ex. Fr) Kummer Stored in Two Different Packaging Materials. Ph.D. Thesis, University of Ghana, Accra, Ghana, July 2015. Avaiable online: http://ugspace.ug.edu.gh:8080/xmlui/handle/123456789/8949 (accessed on 16 July 2016). [Google Scholar]
- Osińska-Jaroszuk, M.; Wlizło, K.; Szałapata, K.; Jarosz-Wilkołazka, A. Correlation between the production of exopolysaccharides and oxalic acid secretion by Ganoderma applanatum and Tyromyces palustris. World J. Microbiol. Biotechnol. 2014, 30, 3065–3074. [Google Scholar] [CrossRef] [PubMed]
- Ogbe, A.O.; Affiku, J.P. Effect of Polyherbal Aqueous Extracts (Moringa oleifera, Gum arabic and wild Ganoderma lucidum) in Comparison with Antibiotic on Growth Performance and Haematological Parameters of Broiler Chickens. Res. J. Recent Sci. 2012, 1, 10–18. [Google Scholar]
- Usman, S.B.; Kyari, S.U.; Abdulrahman, F.I.; Ogbe, A.O.; Ahmad, G.Y.; Ibrahim, U.I.; Sakuma, A.M. Proximate composition, phytochemical and elemental analysis of some organic solvent extract of the wild mushroom—Ganoderma lucidum. J. Nat. Sci. Res. 2012, 2, 24–36. [Google Scholar]
- Takshak, S.; Chaudhary, R.; Sindhu, A.; Singh, A. Biochemical estimation of wild Ganoderma lucidum colleted from differnt agro-climatic zones of Haryana. Int. J. Pharma Biol. Sci. 2014, 5, 143–151. [Google Scholar]
- Ogbe, A.O.; Obeka, A.D. Proximate, Mineral and Anti-Nutrient Composition of Wild Ganoderma lucidum: Implication on Its Utilization in Poultry Production. Iran. J. Appl. Anim. Sci. 2013, 3, 161–166. [Google Scholar]
- Rawat, A.; Mohsin, M.; Sah, A.N.; Negi, P.S.; Singh, S. Biochemical esimation of wildly collected Ganoderma lucidum from Central Himalayan Hills of India. Adv. Appl. Sci. Res. 2012, 3, 3708–3713. [Google Scholar]
- Zhu, F.; Qu, L.; Fan, W.; Qiao, M.; Hao, H.; Wang, X. Assessment of heavy metals in some wild edible mushrooms collected from Yunnan Province, China. Environ. Monit. Assess. 2011, 179, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Dhingra, G.S.; Shri, R. A comparative study of taxonomy, physicochemical parameters, and chemical constituents of Ganoderma lucidum and G. philippii from Uttarakhand, India. Turk. J. Bot. 2014, 38, 186–196. [Google Scholar] [CrossRef]
- Manzi, P.; Aguzzi, A.; Pizzoferrato, L. Nutritional value of mushrooms widely consumed in Italy. Food Chem. 2001, 73, 321–325. [Google Scholar] [CrossRef]
- Stojković, D.S.; Barros, L.; Calhelha, R.C.; Glamočlija, J.; Ćirić, A.; van Griensven, L.J.L.D.; Soković, M.; Ferreira, I.C.F.R. A detailed comparative study between chemical and bioactive properties of Ganoderma lucidum from different origins. Int. J. Food Sci. Nutr. 2014, 65, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Hoa, H.T.; Wang, C.L.; Wang, C.H. The effects of different substrates on the growth, yield, and nutritional composition of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Barros, L.; Martins, A.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Fruiting body, spores and in vitro produced mycelium of Ganoderma lucidum from Northeast Portugal: A comparative study of the antioxidant potential of phenolic and polysaccharidic extracts. Food Res. Int. 2012, 46, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, I.C.F.R.; Barros, L.; Abreu, R.M.V. Antioxidants in wild mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cui, S.W.; Cheung, P.C.K.; Wang, Q. Antitumor polysaccharides from mushrooms: A review on their isolation process, structural characteristics and antitumor activity. Trends Food Sci. Technol. 2007, 18, 4–19. [Google Scholar] [CrossRef]
- Valdez-Morales, M.; Barry, K.; Fahey, G.C.; Domínguez, J.; de Mejia, E.G.; Valverde, M.E.; Paredes-López, O. Effect of maize genotype, developmental stage, and cooking process on the nutraceutical potential of huitlacoche (Ustilago maydis). Food Chem. 2010, 119, 689–697. [Google Scholar] [CrossRef]
- Barros, L.; Baptista, P.; Correia, D.M.; Casal, S.; Oliveira, B.; Ferreira, I.C.F.R. Fatty acid and sugar compositions, and nutritional value of five wild edible mushrooms from Northeast Portugal. Food Chem. 2007, 105, 140–145. [Google Scholar] [CrossRef]
- Martins, A.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Fernandes, I.P.; Barreiro, F.; Ferreira, I.C.F.R. Phenolic extracts of Rubus ulmifolius Schott flowers: Characterization, microencapsulation and incorporation into yogurts as nutraceutical sources. Food Funct. 2014, 5, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.S.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: An inter-species comparative study. Food Chem. Toxicol. 2012, 50, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Barros, L.; Martins, A.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic, polysaccharidic, and lipidic fractions of mushrooms from northeastern portugal: Chemical compounds with antioxidant properties. J. Agric. Food Chem. 2012, 60, 4634–4640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.Y.; Seguin, P.; Ahn, J.K.; Kim, J.J.; Chun, S.C.; Kim, E.H.; Seo, S.H.; Kang, E.Y.; Kim, S.L.; Park, Y.J.; et al. Phenolic Compound Concentration and Antioxidant Activities of Edible and Medicinal Mushrooms from Korea. J. Agric. Food Chem. 2008, 56, 7265–7270. [Google Scholar] [CrossRef]
- Karthikeyan, M.; Radhika, K.; Bhaskaran, R.; Mathiyazhagan, S.; Velazhahan, R. Rapid detection of Ganoderma lucidum and assessment of inhibition effect of various control measures by immunoassay and PCR. Afr. J. Biotechnol. 2009, 8, 2202–2208. [Google Scholar]
- Zhen, H.; Zhang, Z.; Chen, H.; Tian, Z.; Qu, W.; Wu, W. Antioxidant activity of ethanol extract from Ganoderma lucidum cultivated in the medium supplemented with herbs. Acad. J. Med. Plants 2013, 1, 6–13. [Google Scholar] [CrossRef]
- Çaǧlarirmak, N. The nutrients of exotic mushrooms (Lentinula edodes and Pleurotus species) and an estimated approach to the volatile compounds. Food Chem. 2007, 105, 1188–1194. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Park, M.-K. Determination of mineral and trace elements of Ganoderma lucidum consumed in China. J. Environ. Sci. Int. 2007, 16, 21–26. [Google Scholar] [CrossRef]
- Manzi, P.; Gambelli, L.; Marconi, S.; Vivanti, V.; Pizzoferrato, L. Nutrients in edible mushrooms: An inter-species comparative study. Food Chem. 1999, 65, 477–482. [Google Scholar] [CrossRef]
- Duyff, R.L. American Dietetic Association’s Complete Food and Nutrition Guide, 3th ed.; Academy of Nutrition and Dietetics, Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Mhanda, F.N.; Ueitele, I.S.E. Minerals and trace elements in domesticated Namibian Ganoderma species. Afr. J. Biotechnol. 2015, 48, 3216–3218. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA)—Nutrient Database USDA National Nutrient Database for Standard Reference. Available online: http://www.ars.usda.gov/Services/docs (accessed on 17 July 2016).
- Muhammad, A.; Dangoggo, S.M.; Tsafe, A.I.; Itodo, A.U.; Atiku, F.A. Proximate minerals and anti-nutritional factors of Gardenia aqualla (Gauden dutse) fruit pulp. Pak. J. Nutr. 2011, 10, 577–581. [Google Scholar] [CrossRef]
- Stahl, P.D.; Parkin, T.B. Relationship of soil ergosterol concentration and fungal biomass. Soil Biol. Biochem. 1996, 28, 847–855. [Google Scholar] [CrossRef]
- Mattila, P.; Salo-Väänänen, P.; Könkö, K.; Aro, H.; Jalava, T. Basic composition and amino acid contents of mushrooms cultivated in Finland. J. Agric. Food Chem. 2002, 50, 6419–6422. [Google Scholar] [CrossRef] [PubMed]
- Heleno, S.A.; Ferreira, I.C.F.R.; Calhelha, R.C.; Esteves, A.P.; Martins, A.; Queiroz, M.J.R.P. Cytotoxicity of Coprinopsis atramentaria extract, organic acids and their synthesized methylated and glucuronate derivatives. Food Res. Int. 2014, 55, 170–175. [Google Scholar] [CrossRef]
- Yang, J.-H.; Lin, H.-C.; Mau, J.-L. Antioxidant properties of several commercial mushrooms. Food Chem. 2002, 77, 229–235. [Google Scholar] [CrossRef]
- Barros, L.; Falcão, S.; Baptista, P.; Freire, C.; Vilas-Boas, M.; Ferreira, I.C.F.R. Antioxidant activity of Agaricus sp. mushrooms by chemical, biochemical and electrochemical assays. Food Chem. 2008, 111, 61–66. [Google Scholar] [CrossRef]
- Stojković, D.; Reis, F.S.; Barros, L.; Glamočlija, J.; Irić, A.; van Griensven, L.J.I.D.; Soković, M.; Ferreira, I.C.F.R. Nutrients and non-nutrients composition and bioactivity of wild and cultivated Coprinus comatus (O.F.Müll.) Pers. Food Chem. Toxicol. 2013, 59, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, M.; Kalaimagal, C. In vitro antioxidant activity of ethanolic extract of a medicinal mushroom, Ganoderma lucidum. J. Pharm. Sci. Res. 2011, 3, 1427–1433. [Google Scholar]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamurat, T. Antioxidative Properties of Xanthan on the Autoxidation of Soybean Oil in Cyclodextrin Emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists, Ed.; AOAC: Arlington, VA, USA, 1995. [Google Scholar]
- Grangeia, C.; Heleno, S.A.; Barros, L.; Martins, A.; Ferreira, I.C.F.R. Effects of trophism on nutritional and nutraceutical potential of wild edible mushrooms. Food Res. Int. 2011, 44, 1029–1035. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, C.; Ferreira, I.C.F.R. Optimized analysis of organic acids in edible mushrooms from portugal by ultra fast liquid chromatography and photodiode array detection. Food Anal. Methods 2013, 6, 309–316. [Google Scholar] [CrossRef]
- Huang, M.; LaLuzerne, P.; Winters, D.; Sullivan, D. Measurement of vitamin D in foods and nutritional supplements by liquid chromatography/tandem mass spectrometry. J. AOAC Int. 2009, 92, 1327–1335. [Google Scholar] [PubMed]
- Heleno, S.A.; Barros, L.; Sousa, M.J.; Martins, A.; Ferreira, I.C.F.R. Tocopherols composition of Portuguese wild mushrooms with antioxidant capacity. Food Chem. 2010, 119, 1443–1450. [Google Scholar] [CrossRef]
- Sample Availability: Mushroom samples are available from the authors.
| Sample | Nutritional Value | Composition in Sugars | Beta-Glucan (µg/g·dw) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fat | Protein | Ash | Carbohydrates | Energetic Value | Ramnose | Fructose | Mannitol | Sucrose | Trehalose | Total Sugars | ||
| LS1 | 0.78 ± 0.01 f | 24.5 ± 0.3 a | 1.4 ± 0.2 c,d | 73.31 ± 0.08 g | 398.4 ± 0.5 d | 1.63 ± 0.04 b | 1.39 ± 0.05 b | 1.62 ± 0.02 b | 2.85 ± 0.01 b | 1.41 ± 0.01 b | 8.9 ± 0.1 b | 0.98 ± 0.02 f |
| LS2 | 0.95 ± 0.05 d | 23 ± 1 b,c | 0.9 ± 0.1 f | 75.4 ± 0.7 d,e | 401.2 ± 0.2 c | 1.20 ± 0.01 d | 0.42 ± 0.01 d | 0.71 ± 0.04 e | 2.03 ± 0.01 d | 0.77 ± 0.03 c | 5.13 ± 0.05 d | 2.45 ± 0.07 b |
| LS3 | 0.78 ± 0.01 f | 22.5 ± 0.3 c | 1.5 ± 0.1 b,c | 75.2 ± 0.2 d,e,f | 398.0 ± 0.3 d | 0.58 ± 0.01 f | 0.123 ± 0.005 e | 0.28 ± 0.01 h | 1.00 ± 0.03 g | 0.38 ± 0.01 e | 2.37 ± 0.03 f | 1.12 ± 0.03 d |
| LS4 | 0.87 ± 0.01 e | 22.6 ± 0.1 c | 1.47 ± 0.05 b,c | 75.08 ± 0.07 d,e,f | 398.4 ± 0.1 d | 0.56 ± 0.01 f | nd | nd | 1.19 ± 0.06 f | nd | 1.75 ± 0.06 h | 0.80 ± 0.01 g |
| LS5 | 0.91 ± 0.02 d,e | 24 ± 1 a | 0.68 ± 0.01 g | 74.3 ± 0.8 f,g | 401.84 ± 0.07 b,c | 1.49 ± 0.07 c | 1.16 ± 0.01 c | 1.24 ± 0.08 c | 2.61 ± 0.06 c | 1.58 ± 0.07 a | 8.1 ± 0.1 c | 2.67 ± 0.04 b |
| LS6 | 1.16 ± 0.06 c | 22.7 ± 0.1 b,c | 0.9 ± 0.1 f | 75.21 ± 0.07 d,e,f | 402.2 ± 0.5 a,b | 0.87 ± 0.04 e | 0.41 ± 0.04 d | 0.57 ± 0.02 f | 1.50 ± 0.08 e | 0.62 ± 0.03 d | 3.97 ± 0.09 e | 1.83 ± 0.01 c |
| LS7 | 0.53 ± 0.03 g | 23.7 ± 0.2 a,b | 1.19 ± 0.06 d,e | 74.6 ± 0.2 e,f | 397.9 ± 0.3 d | 2.76 ± 0.09 a | 3.05 ± 0.07 a | 2.09 ± 0.03 a | 4.78 ± 0.08 a | 1.37 ± 0.04 b | 14.1 ± 0.1 a | 11.47 ± 0.03 a |
| LS8 | 1.31 ± 0.05 b | 22.2 ± 0.3 c | 1.18 ± 0.08 d,e | 75.3 ± 0.1 d,e | 401.85 ± 0.05 b,c | 0.43 ± 0.02 g | 0.063 ± 0.001 f | 0.15 ± 0.01 i | 0.69 ± 0.02 h | 0.065 ± 0.003 g | 1.39 ± 0.03 i | 2.49 ± 0.08 b |
| LS9 | 1.11 ± 0.03 c | 22.2 ± 0.3 c | 0.88 ± 0.06 f | 75.9 ± 0.3 d | 402.02 ± 0.06 b,c | 0.29 ± 0.02 h | 0.041 ± 0.001 f | 0.16 ± 0.01 i | 0.44 ± 0.03 i | 0.13 ± 0.01 f | 1.06 ± 0.05 j | 1.06 ± 0.06 e |
| DS1 | 0.94 ± 0.05 d | 18.4 ± 0.1 e | 2.12 ± 0.08 a | 78.55 ± 0.01 b | 396.22 ± 0.03 e | 0.42 ± 0.03 g | 0.044 ± 0.003 f | 0.82 ± 0.03 d | 0.72 ± 0.02 h | 0.18 ± 0.04 f | 2.2 ± 0.1 g | tr |
| HS1 | 0.48 ± 0.01 g | 20.4 ± 0.4 d | 1.57 ± 0.08 b | 77.54 ± 0.21 c | 396.1 ± 0.2 e | 0.104 ± 0.001 i | 0.024 ± 0.002 f | 0.063 ± 0.002 j | 0.21 ± 0.01 j | 0.061 ± 0.004 g | 0.457 ± 0.006 l | nd |
| AM1 | 1.40 ± 0.02 a | 15.7 ± 0.1 f | 1.0 ± 0.1 e,f | 81.90 ± 0.01 a | 402.9 ± 0.5 a | 0.155 ± 0.001 i | 0.045 ± 0.002 f | 0.43 ± 0.02 g | 0.249 ± 0.001 j | nd | 0.88 ± 0.02 k | nd |
| LS1 | LS2 | LS3 | LS4 | LS5 | LS6 | LS7 | LS8 | LS9 | DS1 | HS1 | AM1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C6:0 | 0.29 ± 0.01 | 0.108 ± 0.006 | 0.059 ± 0.001 | 0.160 ± 0.004 | 0.161 ± 0.004 | 0.132 ± 0.004 | 0.311 ± 0.002 | 0.111 ± 0.001 | 0.054 ± 0.001 | 0.110 ± 0.001 | 0.058 ± 0.002 | 0.110 ± 0.006 |
| C8:0 | 0.44 ± 0.03 | 0.17 ± 0.01 | 0.115 ± 0.004 | 0.51 ± 0.01 | 0.51 ± 0.01 | 0.140 ± 0.008 | 0.222 ± 0.003 | 0.30 ± 0.02 | 0.164 ± 0.001 | 0.35 ± 0.01 | 0.34 ± 0.01 | 0.19 ± 0.01 |
| C10:0 | 0.57 ± 0.02 | 0.232 ± 0.007 | 0.146 ± 0.005 | 0.36 ± 0.01 | 0.36 ± 0.01 | 0.224 ± 0.003 | 0.36 ± 0.02 | 0.34 ± 0.02 | 0.180 ± 0.004 | 0.32 ± 0.03 | 0.28 ± 0.02 | 0.22 ± 0.01 |
| C12:0 | 0.57 ± 0.01 | 0.30 ± 0.01 | 0.24 ± 0.01 | 0.57 ± 0.03 | 0.58 ± 0.03 | 0.343 ± 0.004 | 0.48 ± 0.02 | 0.45 ± 0.03 | 0.262 ± 0.004 | 0.56 ± 0.06 | 0.556 ± 0.002 | 0.26 ± 0.01 |
| C14:0 | 1.05 ± 0.01 | 0.62 ± 0.03 | 0.60 ± 0.01 | 1.16 ± 0.04 | 1.16 ± 0.04 | 0.73 ± 0.01 | 0.96 ± 0.02 | 0.90 ± 0.02 | 0.56 ± 0.01 | 0.99 ± 0.07 | 1.11 ± 0.02 | 0.59 ± 0.02 |
| C14:1 | 0.52 ± 0.02 | 0.60 ± 0.01 | 0.354 ± 0.002 | 1.21 ± 0.02 | 1.22 ± 0.02 | 0.010 ± 0.001 | 0.019 ± 0.001 | 0.015 ± 0.001 | 0.010 ± 0.001 | 0.173 ± 0.004 | 0.34 ± 0.03 | 0.059 ± 0.004 |
| C15:0 | 1.03 ± 0.01 | 0.95 ± 0.01 | 0.72 ± 0.01 | 0.79 ± 0.01 | 0.79 ± 0.01 | 0.69 ± 0.01 | 0.93 ± 0.02 | 0.44 ± 0.01 | 0.376 ± 0.004 | 0.73 ± 0.06 | 1.03 ± 0.01 | 1.08 ± 0.01 |
| C16:0 | 14.65 ± 0.08 | 15.69 ± 0.12 | 21.73 ± 0.06 | 26.80 ± 0.45 | 26.96 ± 0.41 | 21.52 ± 0.11 | 16.37 ± 0.19 | 23.76 ± 0.11 | 23.72 ± 0.09 | 19.08 ± 0.12 | 23.55 ± 0.06 | 15.89 ± 0.08 |
| C16:1 | 0.28 ± 0.01 | 0.30 ± 0.01 | 0.547 ± 0.004 | 0.82 ± 0.01 | 0.82 ± 0.01 | 0.64 ± 0.01 | 0.32 ± 0.01 | 1.10 ± 0.01 | 1.15 ± 0.02 | 0.301 ± 0.004 | 0.419 ± 0.005 | 0.437 ± 0.002 |
| C17:0 | 1.13 ± 0.01 | 1.19 ± 0.07 | 1.49 ± 0.01 | 1.66 ± 0.01 | 1.67 ± 0.02 | 1.46 ± 0.01 | 1.16 ± 0.01 | 1.32 ± 0.01 | 1.18 ± 0.01 | 1.34 ± 0.07 | 2.63 ± 0.01 | 1.03 ± 0.01 |
| C18:0 | 5.68 ± 0.05 | 6.09 ± 0.03 | 7.63 ± 0.03 | 12.27 ± 0.04 | 12.35 ± 0.06 | 8.58 ± 0.02 | 6.39 ± 0.04 | 8.89 ± 0.03 | 8.56 ± 0.01 | 9.91 ± 0.33 | 16.03 ± 0.09 | 5.70 ± 0.02 |
| C18:1n9 | 21.78 ± 0.07 | 25.97 ± 0.10 | 39.44 ± 0.02 | 35.03 ± 0.09 | 35.25 ± 0.02 | 42.28 ± 0.03 | 26.19 ± 0.10 | 45.80 ± 0.15 | 48.76 ± 0.14 | 24.82 ± 0.29 | 22.55 ± 0.14 | 28.81 ± 0.04 |
| C18:2n6 | 44.27 ± 0.09 | 40.09 ± 0.09 | 21.83 ± 0.01 | 9.74 ± 0.12 | 9.80 ± 0.14 | 17.53 ± 0.01 | 40.96 ± 0.04 | 7.57 ± 0.01 | 7.48 ± 0.02 | 30.55 ± 0.23 | 17.12 ± 0.08 | 40.43 ± 0.12 |
| C18:3n3 | 0.247 ± 0.004 | 0.23 ± 0.01 | 0.153 ± 0.005 | 0.167 ± 0.007 | 0.168 ± 0.007 | 0.140 ± 0.001 | 0.30 ± 0.02 | 0.210 ± 0.004 | 0.43 ± 0.02 | 0.33 ± 0.02 | 0.491 ± 0.003 | 0.34 ± 0.02 |
| C20:0 | 0.44 ± 0.02 | 0.44 ± 0.02 | 0.69 ± 0.04 | 1.14 ± 0.05 | 1.15 ± 0.04 | 0.77 ± 0.01 | 0.44 ± 0.01 | 0.799 ± 0.001 | 0.86 ± 0.01 | 0.77 ± 0.01 | 1.14 ± 0.10 | 0.36 ± 0.03 |
| C20:1 | 0.095 ± 0.001 | 0.117 ± 0.008 | 0.081 ± 0.006 | 0.131 ± 0.001 | 0.132 ± 0.001 | 0.082 ± 0.004 | 0.123 ± 0.006 | 0.086 ± 0.001 | 0.055 ± 0.001 | 0.053 ± 0.004 | nd | 0.120 ± 0.001 |
| C20:2 | 0.057 ± 0.004 | 0.051 ± 0.004 | 0.061 ± 0.001 | 0.076 ± 0.006 | 0.076 ± 0.006 | 0.073 ± 0.006 | 0.012 ± 0.002 | 0.064 ± 0.002 | 0.056 ± 0.001 | 0.060 ± 0.001 | nd | 0.038 ± 0.002 |
| C20:3 | 0.26 ± 0.01 | 0.35 ± 0.03 | 0.215 ± 0.001 | 0.48 ± 0.05 | 0.48 ± 0.05 | 0.177 ± 0.001 | 0.29 ± 0.02 | 0.118 ± 0.002 | 0.090 ± 0.001 | 0.38 ± 0.02 | 0.40 ± 0.01 | 0.23 ± 0.02 |
| C20:5n3 | 0.27 ± 0.01 | 0.129 ± 0.001 | 0.227 ± 0.002 | 0.36 ± 0.01 | 0.37 ± 0.01 | nd | 0.206 ± 0.003 | 0.353 ± 0.001 | 0.35 ± 0.01 | 0.142 ± 0.001 | 0.99 ± 0.01 | 0.18 ± 0.01 |
| C22:0 | 1.58 ± 0.05 | 1.76 ± 0.14 | 1.29 ± 0.03 | 3.09 ± 0.20 | 2.50 ± 0.02 | 1.20 ± 0.06 | 1.34 ± 0.02 | 1.27 ± 0.01 | 0.99 ± 0.05 | 1.55 ± 0.03 | 2.26 ± 0.06 | 1.16 ± 0.01 |
| C22:1n9 | 0.35 ± 0.01 | 0.34 ± 0.04 | 0.118 ± 0.002 | 0.44 ± 0.04 | 0.45 ± 0.04 | 0.22 ± 0.01 | 0.35 ± 0.03 | 0.27 ± 0.01 | 0.282 ± 0.003 | 0.66 ± 0.06 | 0.72 ± 0.02 | 0.21 ± 0.01 |
| C23:0 | 0.72 ± 0.02 | 1.07 ± 0.02 | 0.773 ± 0.002 | 1.19 ± 0.07 | 1.20 ± 0.07 | 0.58 ± 0.01 | 0.52 ± 0.01 | 0.93 ± 0.01 | 1.03 ± 0.01 | 1.07 ± 0.01 | 1.79 ± 0.04 | 0.87 ± 0.01 |
| C24:0 | 3.73 ± 0.27 | 3.22 ± 0.11 | 1.50 ± 0.02 | 1.85 ± 0.18 | 1.86 ± 0.19 | 2.49 ± 0.06 | 1.78 ± 0.05 | 4.91 ± 0.03 | 3.40 ± 0.07 | 5.75 ± 0.06 | 6.20 ± 0.10 | 1.67 ± 0.02 |
| Total SFA (% of total FA) | 31.9 ± 0.2 h | 31.8 ± 0.2 h | 36.98 ± 0.01 g | 51.6 ± 0.1 b | 51.25 ± 0.19 b | 38.9 ± 0.1 f | 31.3 ± 0.2 i | 44.4 ± 0.1 c | 41.3 ± 0.1 e | 42.5 ± 0.6 d | 57 ± 1 a | 29.1 ± 0.1 j |
| Total MUFA (% of total FA) | 23.0 ± 0.1 j | 27.3 ± 0.1 g | 40.54 ± 0.01 d | 37.6 ± 0.1 e | 37.87 ± 0.01 e | 43.2 ± 0.1 c | 27.0 ± 0.1 g | 47.3 ± 0.2 b | 50.3 ± 0.1 a | 26.0 ± 0.3 h | 24.0 ± 0.1 i | 29.64 ± 0.02 f |
| Total PUFA (% of total FA) | 45.1 ± 0.1 a | 40.9 ± 0.1 d | 22.49 ± 0.01 f | 10.8 ± 0.2 i | 10.88 ± 0.20 i | 17.9 ± 0.1 h | 41.8 ± 0.1 b | 8.31 ± 0.01 j | 8.41 ± 0.01 j | 31.5 ± 0.3 e | 19.0 ± 0.1 g | 41.2 ± 0.1 c |
| Sample | Phenolic Compounds | Other Organic Compounds | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Protocatechuic Acid | p-Hydroxybenzoic Acid | p-Coumaric Acid | Total Phenolic Compounds | Cinamic Acid | Oxalic Acid | Malic Acid | Fumaric Acid | Total Organic Compounds | |
| LS1 | 14 ± 2 f | nd | nd | 14 ± 2 j | 15.8 ± 0.2 e,f | 111 ± 1 f | 88.29 ± 0.04 a | 8.4 ± 0.2 a | 208 ± 2 d |
| LS2 | 65.6 ± 0.1 b | 57.9 ± 0.2 f | 9.4 ± 0.1 d | 132.9 ± 0.2 e | 19 ± 4 d,e | 125.3 ± 0.3 d | 64 ± 1 e | 7.92 ± 0.01 a | 198 ± 1 e |
| LS3 | 7.6 ± 0.2 g | nd | nd | 7.6 ± 0.2 k | 8.3 ± 0.2 g | 546 ± 1 b | 39 ± 1 g | 0.13 ± 0.02 c | 586 ± 1 b |
| LS4 | nd | nd | nd | - | 14 ± 2 f | 1003 ± 1 a | nd | nd | 1003 ± 1 a |
| LS5 | 13.7 ± 0.3 f | 22.7 ± 0.1 h | nd | 36.4 ± 0.4 i | 12.7 ± 0.4 f | 145 ± 1 c | 81 ± 3 b | 3.06 ± 0.05 b | 229 ± 2 c |
| LS6 | 87 ± 1 a | 126 ± 4 c | 3.8 ± 0.1 g | 217 ± 5 c | 50.4 ± 0.4 b | 85 ± 1 h | 86.6 ± 0.2 a | 0.5 ± 0.1 c | 172 ± 1 h |
| LS7 | nd | 107 ± 1 d | nd | 107 ± 1 f | 51 ± 4 b | 101.4 ± 0.5 g | 73 ± 1 c | 8 ± 1 a | 183 ± 2 g |
| LS8 | 55 ± 2 d | 126 ± 3 c | 19.9 ± 0.2 b | 201 ± 1 d | 48.7 ± 0.9 b | 112.3 ± 0.4 f | 38 ± 1 g | nd | 150 ± 1 i |
| LS9 | 50.8 ± 0.2 e | 34.7 ± 0.3 g | 15.1 ± 0.2 c | 101 ± 1 g | 21.5 ± 0.3 d | 119 ± 1 e | 67 ± 1 d | nd | 186.1 ± 0.4 f |
| DS1 | 59 ± 1 c | 176 ± 1 b | 7.0 ± 0.1 e | 242 ± 1 b | 39.5 ± 0.6 c | 79 ± 2 i | 57 ± 1 f | nd | 135.8 ± 0.5 j |
| HS1 | 16.6 ± 0.3 f | 67 ± 1 e | 6.5 ± 0.1 f | 90 ± 1 h | 1.2 ± 0.1 h | 52.2 ± 0.1 j | 22 ± 1 h | 3.61 ± 0.01 b | 78 ± 1 k |
| AM1 | 85 ± 4 a | 331 ± 7 a | 73 ± 1 a | 489 ± 4 a | 234.8 ± 1.9 a | 51.7 ± 0.1 j | 22 ± 1 h | 3.57 ± 0.01 b | 77 ± 1 k |
| Component | LS1 | LS2 | LS3 | LS4 | LS5 | LS6 | LS7 | LS8 | LS9 | DS1 | HS1 | AM1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Major mineral elements | P | 167 ± 1 d | 193.6 ± 0.8 b | 106.1 ± 0.3 f | 186 ± 1 c | 102.4 ± 0.6 g | 37.7 ± 0.1 j | 129.0 ± 0.2 e | 49.1 ± 0.1 i | 79.7 ± 0.1 h | 472 ± 2 a | nd | nd |
| K | 438.6 ± 0.4 d | 471.4 ± 0.9 b | 371 ± 1 f | 637.9 ± 0.2 a | 273.2 ± 0.5 g | 226.9 ± 0.2 h | 429.5 ± 0.7 e | 100 ± 2 j | 187 ± 1 i | 452.4 ± 0.7 c | nd | nd | |
| Ca | 83.0 ± 0.4 f | 141.6 ± 0.5 c | 75.2 ± 0.2 h | 80 ± 1 g | 83.6 ± 0.3 f | 124 ± 1 d | 56.8 ± 0.7 i | 96.4 ± 0.4 e | 147.8 ± 0.3 b | 248 ± 1 a | nd | nd | |
| Mg | 56.1 ± 1.4 g | 71.5 ± 0.1 e | 66.4 ± 0.2 f | 73.9 ± 0.3 d | 55 ± 1 g | 102.9 ± 0.8 b | 48 ± 1 h | 43 ± 1 i | 93 ± 1 c | 204.8 ± 0.2 a | nd | nd | |
| Na | 0.838 ± 0.003 h | 0.89 ± 0.03 h | 0.913 ± 0.004 h | 3.43 ± 0.04 d | 1.03 ± 0.03 g | 3.7 ± 0.1 c | 1.57 ± 0.01 f | 2.57 ± 0.06 e | 4.37 ± 0.04 b | 4.53 ± 0.01 a | nd | nd | |
| S | 147 ± 3 d | 165.6 ± 0.2 b | 155 ± 2 c | 169 ± 1 b | 144 ± 1 de | 119 ± 2 g | 128 ± 4 f | 140 ± 3 e | 111.2 ± 0.4 h | 187 ± 2 a | nd | nd | |
| Trace elements | Al | 7.6 ± 0.1 e,f | 3.07 ± 0.03 g,h | 35.80 ± 0.04 c | 9.30 ± 0.04 e | 4.80 ± 0.07 f,g,h | 67 ± 4 b | 2.38 ± 0.03 h | 26.9 ± 0.4 d | 246 ± 4 a | 7.0 ± 0.1 ef | nd | nd |
| B | 0.49 ± 0.01 c | 0.49 ± 0.03 c | <0.014 e | <0.014 e | 0.54 ± 0.01 b | 0.23 ± 0.01 d | 0.74 ± 0.01 a | 0.26 ± 0.01 d | <0.014 e | 0.52 ± 0.03 bc | nd | nd | |
| Cu | 0.68 ± 0.01 c,d | 0.60 ± 0.02 d | 0.49 ± 0.04 e | 1.05 ± 0.01 b | 0.45 ± 0.05 e | 0.44 ± 0.01 e | 0.73 ± 0.06 c | 0.29 ± 0.02 f | 0.34 ± 0.04 f | 3.2 ± 0.1 a | nd | nd | |
| Fe | 10.5 ± 0.1 e,f | 8.2 ± 0.2 f | 29.1 ± 0.1 d | 10.9 ± 0.4 e | 8.6 ± 0.5 ef | 57.64 ± 3.03 b | 4.99 ± 0.09 g | 35.1 ± 0.2 c | 299 ± 2 a | 8.88 ± 0.06 ef | nd | nd | |
| Mn | 2.0 ± 0.2 b | 1.58 ± 0.08 d,e | 1.86 ± 0.03 bc | 1.83 ± 0.06 c | 1.73 ± 0.03 cd | 1.43 ± 0.06 e | 0.56 ± 0.03 g | 1.46 ± 0.07 e | 4.22 ± 0.06 a | 1.12 ± 0.01 f | nd | nd | |
| Mo | <0.029 | <0.029 | <0.029 | <0.029 | <0.029 | <0.029 | <0.029 | <0.029 | <0.029 | 0.072 ± 0.001 | nd | nd | |
| Zn | 1.64 ± 0.04 c | 2.0 ± 0.1 b | 1.61 ± 0.08 c | 3.06 ± 0.01 a | 1.14 ± 0.01 e | 0.71 ± 0.01 f | 1.46 ± 0.04 d | 0.56 ± 0.04 g | 1.18 ± 0.01 e | 1.38 ± 0.03 d | nd | nd | |
| Vitamins | Ergosterol | 296 ± 6 d | 373 ± 2 a | 310 ± 6 c | 288 ± 3 d | 328 ± 1 b | 113 ± 4 g | 308 ± 1 c | 237 ± 3 e | 331 ± 3 b | 151 ± 6 f | nd | nd |
| Vitamin D2 | 4692 ± 57 d | 4338 ± 42 e | 3389 ± 14 g | 6636 ± 17 b | 7158 ± 19 a | 891 ± 18 i | 4904 ± 13 c | 3660 ± 21 f | 3129 ± 14 h | nd | nd | nd | |
| α-tocopherol | 22 ± 1 c | 22 ± 1 c | 19 ± 1 d | nd | 55 ± 0.5 a | 38 ± 1 b | 16 ± 2 d | 17 ± 1 d | 18 ± 2 d | 53 ± 3 a | 18 ± 1 d | 9.45 ± 0.02 e | |
| β-tocopherol | 719 ± 1 l | 1730 ± 2 e | 2653 ± 1 b | 2592 ± 1 c | 3136 ± 12 a | 1860 ± 2 d | 1137 ± 2 j | 1360 ± 2 h | 1660 ± 3 f | 1295 ± 1 i | 1369 ± 2 g | 796 ± 4 k | |
| Total tocopherols | 741 ± 2 k | 1752 ± 3 e | 2671 ± 1 b | 2592 ± 1 c | 3191 ± 12 a | 1898 ± 3 d | 1153 ± 3 i | 1378 ± 3 g | 1678 ± 5 f | 1349 ± 2 h | 1386 ± 3 g | 806 ± 4 j |
| Sample | Reducing Power | Radical Scavenging Activity | Lipid Peroxidation Inhibition | ||
|---|---|---|---|---|---|
| Folin-Ciocalteu (mg GAE/g Extract) | Ferricyanide/Prussian Blue (EC50; mg/mL) | DPPH Scavenging Activity (EC50; mg/mL) | β-Carotene/Linoleate (EC50; mg/mL) | TBARS (EC50; mg/mL) | |
| LS1 | 53.5 ± 0.4 i | 0.85 ± 0.01 c | 2.26 ± 0.03 c | 1.5 ± 0.1 c | 0.36 ± 0.01 b |
| LS2 | 79 ± 1 e | 0.34 ± 0.01 g | 1.52 ± 0.04 e,f | 1.00 ± 0.05 e,f | 0.24 ± 0.03 e |
| LS3 | 35.4 ± 0.1 j | 0.96 ± 0.01 b | 4.1 ± 0.2 b | 1.9 ± 0.1 b | 0.36 ± 0.04 b |
| LS4 | 24.3 ± 0.2 k | 1.07 ± 0.01 a | 4.5 ± 0.1 a | 2.2 ± 0.1 a | 0.44 ± 0.01 a |
| LS5 | 63.7 ± 0.6 h | 0.67 ± 0.01 d | 1.7 ± 0.1 d | 1.33 ± 0.02 c,d | 0.28 ± 0.01 c |
| LS6 | 101 ± 1 c | 0.24 ± 0.01 i | 1.02 ± 0.04 g | 0.90 ± 0.04 f | 0.22 ± 0.01 e,f |
| LS7 | 75 ± 2 f | 0.43 ± 0.01 f | 1.56 ± 0.02 e | 1.04 ± 0.05 e | 0.24 ± 0.03 e |
| LS8 | 86 ± 1 d | 0.27 ± 0.01 h | 1.42 ± 0.01 f | 0.9 ± 0.1 e,f | 0.22 ± 0.03 d |
| LS9 | 70 ± 1 g | 0.44 ± 0.01 f | 1.57 ± 0.04 e | 1.23 ± 0.02 d | 0.26 ± 0.03 c,d |
| DS1 | 124 ± 1 b | 0.23 ± 0.01 i | 0.38 ± 0.01 h | 0.90 ± 0.04 f | 0.19 ± 0.01 f |
| HS1 | 70 ± 1 g | 0.64 ± 0.02 e | 1.59 ± 0.04 e | 1.3 ± 0.2 d | 0.35 ± 0.03 b |
| AM1 | 147 ± 2 a | 0.19 ± 0.01 j | 0.38 ± 0.01 h | 0.50 ± 0.03 g | 0.100 ± 0.002 g |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obodai, M.; Narh Mensah, D.L.; Fernandes, Â.; Kortei, N.K.; Dzomeku, M.; Teegarden, M.; Schwartz, S.J.; Barros, L.; Prempeh, J.; Takli, R.K.; et al. Chemical Characterization and Antioxidant Potential of Wild Ganoderma Species from Ghana. Molecules 2017, 22, 196. https://doi.org/10.3390/molecules22020196
Obodai M, Narh Mensah DL, Fernandes Â, Kortei NK, Dzomeku M, Teegarden M, Schwartz SJ, Barros L, Prempeh J, Takli RK, et al. Chemical Characterization and Antioxidant Potential of Wild Ganoderma Species from Ghana. Molecules. 2017; 22(2):196. https://doi.org/10.3390/molecules22020196
Chicago/Turabian StyleObodai, Mary, Deborah L. Narh Mensah, Ângela Fernandes, Nii Korley Kortei, Matilda Dzomeku, Matthew Teegarden, Steven J. Schwartz, Lillian Barros, Juanita Prempeh, Richard K. Takli, and et al. 2017. "Chemical Characterization and Antioxidant Potential of Wild Ganoderma Species from Ghana" Molecules 22, no. 2: 196. https://doi.org/10.3390/molecules22020196
APA StyleObodai, M., Narh Mensah, D. L., Fernandes, Â., Kortei, N. K., Dzomeku, M., Teegarden, M., Schwartz, S. J., Barros, L., Prempeh, J., Takli, R. K., & Ferreira, I. C. F. R. (2017). Chemical Characterization and Antioxidant Potential of Wild Ganoderma Species from Ghana. Molecules, 22(2), 196. https://doi.org/10.3390/molecules22020196