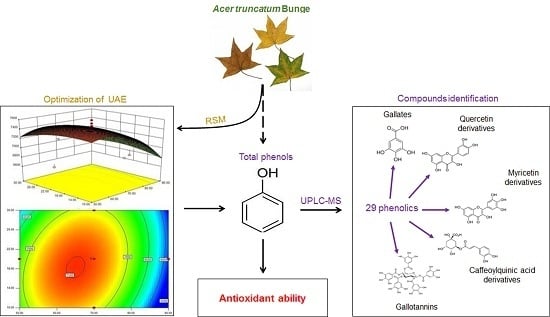
Response Surface Methodology Optimization of Ultrasonic-Assisted Extraction of Acer Truncatum Leaves for Maximal Phenolic Yield and Antioxidant Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of Solvents and Independent Variables on UAE
2.1.1. Solvent Types
2.1.2. Solvent Concentration
2.1.3. Material-to-Liquid Ratio
2.1.4. Extraction Temperature
2.1.5. Ultrasonic Power
2.1.6. Extraction Time
2.2. Statistical Analysis and Model Fitting
2.3. Effect of Interactions Among Variables on Phenolic Yield and Antioxidant Activity in ATL
2.4. Characterization of Phenolic Compositions in ATL
2.4.1. Gallates and Gallotannins Derivatives
2.4.2. Flavonoids
2.4.3. Chlorogenic Acid Derivatives
2.4.4. Other Phenolic Compounds
3. Materials and Methods
3.1. Chemicals and Plant Materials
3.2. Optimization of UAE
3.2.1. Preliminary Experiments
3.2.2. RSM Experiment
3.3. Measurement of Total Phenols
3.4. Determination of DPPH• Scavenging Activity
3.5. UPLC-QTOF-MS/MS Analyses of Phenolic Compositions
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guo, X.; Wang, R.; Chang, R.; Liang, X.; Wang, C.; Luo, Y.; Yuan, Y.; Guo, W. Effects of nitrogen addition on growth and photosynthetic characteristics of Acer truncatum seedlings. Dendrobiology 2014, 72, 151–161. [Google Scholar] [CrossRef]
- More, D.; White, J. Cassell’s Trees of Britain and Northern Europe; Cassell: London, UK, 2003. [Google Scholar]
- Ma, X.; Tian, W.; Wu, L.; Cao, X.; Ito, Y. Isolation of quercetin-3-O-l-rhamnoside from Acer truncatum Bunge by high-speed counter-current chromatography. J. Chromatogr. A 2005, 1070, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wu, L.; Ito, Y.; Tian, W. Application of preparative high-speed counter-current chromatography for separation of methyl gallate from Acer truncatum Bunge. J. Chromatogr. A 2005, 1076, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wu, X.D.; You, X.F.; Ma, X.F.; Tian, W.X. Inhibitory effects on bacterial growth and beta-ketoacyl-ACP reductase by different species of maple leaf extracts and tannic acid. Phytother. Res. 2010, 24, S35–S41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Luo, S.Y.; Ye, Y.B.; Zhao, W.H.; Sun, X.G.; Wang, Z.Q.; Li, R.; Sun, Y.H.; Tian, W.X.; Zhang, Y.X. The antibacterial efficacy of an aceraceous plant [Shantung maple (Acer truncatum Bunge)] may be related to inhibition of bacterial β-oxoacyl-acyl carrier protein reductase (FabG). Biotechnol. Appl. Biochem. 2008, 51, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.H.; Gao, C.; Zhang, Y.X.; Tian, W.X. Evaluation of the inhibitory activities of aceraceous plants on fatty acid synthase. J. Enzyme Inhib. Med. Chem. 2007, 22, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.H.; Zhang, J.F.; Zhe, W.; Zhang, Y.X.; Tian, W.X. The extract of leaves of Acer truncatum Bunge: A natural inhibitor of fatty acid synthase with antitumor activity. J. Enzyme Inhib. Med. Chem. 2006, 21, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Zill-e, H.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Huang, J.; Wu, G.; Tong, J.; Xie, G.; Duan, J.-A.; Qin, M. Multiple responses optimization of ultrasonic-assisted extraction by response surface methodology (RSM) for rapid analysis of bioactive compounds in the flower head of Chrysanthemum morifolium Ramat. Ind. Crop. Prod. 2015, 74, 192–199. [Google Scholar] [CrossRef]
- Jerman, T.; Trebše, P.; Mozetič Vodopivec, B. Ultrasound-assisted solid liquid extraction (USLE) of olive fruit (Olea europaea) phenolic compounds. Food Chem. 2010, 123, 175–182. [Google Scholar] [CrossRef]
- Tchabo, W.; Ma, Y.; Engmann, F.N.; Zhang, H. Ultrasound-assisted enzymatic extraction (UAEE) of phytochemical compounds from mulberry (Morus nigra) must and optimization study using response surface methodology. Ind. Crop. Prod. 2015, 63, 214–225. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Liang, H.; Zhang, Q.; Li, Q. Optimization of ionic liquid based ultrasonic assisted extraction of antioxidant compounds from Curcuma longa L. using response surface methodology. Ind. Crop. Prod. 2015, 76, 487–493. [Google Scholar] [CrossRef]
- Xu, G.; Liang, C.; Huang, P.; Liu, Q.; Xu, Y.; Ding, C.; Li, T. Optimization of rice lipid production from ultrasound-assisted extraction by response surface methodology. J. Cereal Sci. 2016, 70, 23–28. [Google Scholar] [CrossRef]
- Wu, H.; Zhu, J.; Diao, W.; Wang, C. Ultrasound-assisted enzymatic extraction and antioxidant activity of polysaccharides from pumpkin (Cucurbita moschata). Carbohyd. Polym. 2014, 113, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, H.; Liu, W.; Pei, J.; Wang, Z.; Zhou, H.; Yan, J. Ultrasound enhanced production and antioxidant activity of polysaccharides from mycelial fermentation of Phellinus igniarius. Carbohyd. Polym. 2014, 113, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Flores, M.; Germán-Báez, L.J.; Gutiérrez-Dorado, R.; Medina-Godoy, S.; Norzagaray-Valenzuela, C.; Hernández-Verdugo, S.; Reyes-Moreno, C.; Valdez-Ortiz, A. Improving bioactivities of Jatropha curcas protein hydrolysates by optimizing with response surface methodology the extrusion cooking process. Ind. Crop. Prod. 2016, 85, 353–360. [Google Scholar] [CrossRef]
- Ma, C.H.; Liu, T.T.; Yang, L.; Zu, Y.G.; Wang, S.Y.; Zhang, R.R. Study on ionic liquid-based ultrasonic-assisted extraction of biphenyl cyclooctene lignans from the fruit of Schisandra chinensis Baill. Anal. Chim. Acta 2011, 689, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Hatambeygi, N.; Abedi, G.; Talebi, M. Method development and validation for optimised separation of salicylic, acetyl salicylic and ascorbic acid in pharmaceutical formulations by hydrophilic interaction chromatography and response surface methodology. J. Chromatogr. A 2011, 1218, 5995–6003. [Google Scholar] [CrossRef] [PubMed]
- Talebpour, Z.; Ghassempour, A.; Abbaci, M.; Aboul-Enein, H.Y. Optimization of microwave-assisted extraction for the determination of glycyrrhizin in menthazin herbal drug by experimental design methodology. Chromatographia 2009, 70, 191–197. [Google Scholar] [CrossRef]
- Wang, T.; Liang, H.; Yuan, Q. Optimization of ultrasonic-stimulated solvent extraction of sinigrin from Indian mustard seed (Brassica juncea L.) using response surface methodology. Phytochem. Anal. 2011, 22, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Dayana Priyadharshini, S.; Bakthavatsalam, A.K. Optimization of phenol degradation by the microalga Chlorella pyrenoidosa using Plackett-Burman Design and Response Surface Methodology. Bioresour. Technol. 2016, 207, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Elboughdiri, N.; Mahjoubi, A.; Shawabkeh, A.; Khasawneh, H.E.; Jamoussi, B. Optimization of the degradation of hydroquinone, resorcinol and catechol using response surface methodology. Adv. Chem. Eng. Sci. 2015, 5, 111–120. [Google Scholar] [CrossRef]
- Samavati, V. Polysaccharide extraction from Abelmoschus esculentus: Optimization by response surface methodology. Carbohyd. Polym. 2013, 95, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Vahabzadeh, F.; Bonakdarpour, B.; Mehranian, M. Empirical modeling of olive oil mill wastewater treatment using loofa-immobilized Phanerochaete chrysosporium. Process Biochem. 2006, 41, 1148–1154. [Google Scholar] [CrossRef]
- Barnaba, C.; Dellacassa, E.; Nicolini, G.; Nardin, T.; Malacarne, M.; Larcher, R. Identification and quantification of 56 targeted phenols in wines, spirits, and vinegars by online solid-phase extraction—Ultrahigh-Performance Liquid Chromatography—Quadrupole-Orbitrap mass spectrometry. J. Chromatogr. A 2015, 1423, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Díaz-de-Cerio, E.; Gómez-Caravaca, A.M.; Verardo, V.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Determination of guava (Psidium guajava L.) leaf phenolic compounds using HPLC-DAD-QTOF-MS. J. Funct. Foods 2016, 22, 376–388. [Google Scholar] [CrossRef]
- Heras, R.M.-L.; Quifer-Rada, P.; Andrés, A.; Lamuela-Raventós, R. Polyphenolic profile of persimmon leaves by high resolution mass spectrometry (LC-ESI-LTQ-Orbitrap-MS). J. Funct. Foods 2016, 23, 370–377. [Google Scholar] [CrossRef]
- Melguizo-Melguizo, D.; Diaz-de-Cerio, E.; Quirantes-Piné, R.; Švarc-Gajić, J.; Segura-Carretero, A. The potential of Artemisia vulgaris leaves as a source of antioxidant phenolic compounds. J. Funct. Foods 2014, 10, 192–200. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J. Identification and quantification of polyphenolic compounds in ten pear cultivars by UPLC-PDA-Q/TOF-MS. J. Food Compos. Anal. 2016, 49, 65–77. [Google Scholar] [CrossRef]
- Fu, Z.-F.; Tu, Z.-C.; Zhang, L.; Wang, H.; Wen, Q.-H.; Huang, T. Antioxidant activities and polyphenols of sweet potato (Ipomoea batatas L.) leaves extracted with solvents of various polarities. Food Biosci. 2016, 15, 11–18. [Google Scholar] [CrossRef]
- Metrouh-Amir, H.; Duarte, C.M.M.; Maiza, F. Solvent effect on total phenolic contents, antioxidant, and antibacterial activities of Matricaria pubescens. Ind. Crop. Prod. 2015, 67, 249–256. [Google Scholar] [CrossRef]
- Wijekoon, M.M.J.O.; Bhat, R.; Karim, A.A. Effect of extraction solvents on the phenolic compounds and antioxidant activities of bunga kantan (Etlingera elatior Jack.) inflorescence. J. Food Compos. Anal. 2011, 24, 615–619. [Google Scholar] [CrossRef]
- Cujic, N.; Savikin, K.; Jankovic, T.; Pljevljakusic, D.; Zdunic, G.; Ibric, S. Optimization of polyphenols extraction from dried chokeberry using maceration as traditional technique. Food Chem. 2016, 194, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Belwal, T.; Dhyani, P.; Bhatt, I.D.; Rawal, R.S.; Pande, V. Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM). Food Chem. 2016, 207, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, S.V.; Ol’khovich, M.V.; Sharapova, A.V.; Proshin, A.N.; Perlovich, G.L. Partition coefficients and thermodynamics of transfer of novel drug-like spiro-derivatives in model biological solutions. J. Chem. Thermodyn. 2013, 61, 11–17. [Google Scholar] [CrossRef]
- Filgueiras, A.V.; Capelo, J.L.; Lavilla, I.; Bendicho, C. Comparison of ultrasound-assisted extraction and microwave-assisted digestion for determination of magnesium, manganese and zinc in plant samples by flame atomic absorption spectrometry. Talanta 2000, 53, 433–441. [Google Scholar] [CrossRef]
- Toma, M.; Vinatoru, M.; Paniwnyk, L.; Mason, T.J. Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason. Sonochem. 2001, 8, 137–142. [Google Scholar] [CrossRef]
- Entezari, M.H.; Kruus, P. Effect of frequency on sonochemical reactions II. Temperature and intensity effects. Ultrason. Sonochem. 1996, 3, 19–24. [Google Scholar] [CrossRef]
- Raso, J.; Mañas, P.; Pagán, R.; Sala, F.J. Influence of different factors on the output power transferred into medium by ultrasound. Ultrason. Sonochem. 1999, 5, 157–162. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, Z.; Zheng, B.; Martin Lo, Y. Optimization of ultrasonic-assisted extraction of pomegranate (Punica granatum L.) seed oil. Ultrason. Sonochem. 2013, 20, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, D.; Chen, J.; Ye, X.; Yu, D. Effects of different factors of ultrasound treatment on the extraction yield of the all-trans-beta-carotene from citrus peels. Ulcatrason. Sonochem. 2011, 18, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Hemwimol, S.; Pavasant, P.; Shotipruk, A. Ultrasound-assisted extraction of anthraquinones from roots of Morinda citrifolia. Ultrason. Sonochem. 2006, 13, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Wang, H.; Zhang, M.; Wang, Z. Improved extraction of oil from chickpea under ultrasound in a dynamic system. J. Food Eng. 2010, 98, 13–18. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Tan, M.C.; Tan, C.P.; Ho, C.W. Effects of extraction solvent system, time and temperature on total phenolic content of henna (Lawsonia inermis) stems. Int. Food Res. J. 2013, 20, 3117–3123. [Google Scholar]
- Li, Y.; Fabiano-Tixier, A.S.; Tomao, V.; Cravotto, G.; Chemat, F. Green ultrasound-assisted extraction of carotenoids based on the bio-refinery concept using sunflower oil as an alternative solvent. Ultrason. Sonochem. 2013, 20, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Erbay, Z.; Icier, F. Optimization of hot air drying of olive leaves using response surface methodology. J. Food Eng. 2009, 91, 533–541. [Google Scholar] [CrossRef]
- Jin, X.; Ning, Y. Extraction optimization and bioactivity of polysaccharides from Aspergillus fumigatus AF1. Carbohyd. Polym. 2013, 96, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Prakash Maran, J.; Mekala, V.; Manikandan, S. Modeling and optimization of ultrasound-assisted extraction of polysaccharide from Cucurbita moschata. Carbohyd. Polym. 2013, 92, 2018–2026. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Li, J.; Zou, H. Study on extraction of polyphenols from Acer truncatum with microwave. Zhong Yao Cai 2008, 31, 1728–1730, (With abstract in English and main text in Chinese). [Google Scholar] [PubMed]
- Yang, L.; Yin, P.; Li, K.; Fan, H.; Xue, Q.; Li, X.; Sun, L.; Liu, Y. Seasonal dynamics of constitutive levels of phenolic components lead to alterations of antioxidant capacities in Acer truncatum leaves. Arab. J. Chem. 2017. [Google Scholar] [CrossRef]
- Dorta, E.; González, M.; Lobo, M.G.; Sánchez-Moreno, C.; de Ancos, B. Screening of phenolic compounds in by-product extracts from mangoes (Mangifera indica L.) by HPLC-ESI-QTOF-MS and multivariate analysis for use as a food ingredient. Food Res. Int. 2014, 57, 51–60. [Google Scholar] [CrossRef]
- Kiss, A.K.; Naruszewicz, M. Polyphenolic compounds characterization and reactive nitrogen species scavenging capacity of Oenothera paradoxa defatted seed extracts. Food Chem. 2012, 131, 485–492. [Google Scholar] [CrossRef]
- Grundhöfer, P.; Niemetz, R.; Schilling, G.; Gross, G.G. Biosynthesis and subcellular distribution of hydrolyzable tannins. Phytochemistry 2001, 57, 915–927. [Google Scholar] [CrossRef]
- Mocan, A.; Schafberg, M.; Crișan, G.; Rohn, S. Determination of lignans and phenolic components of Schisandra chinensis (Turcz.) Baill. using HPLC-ESI-ToF-MS and HPLC-online TEAC: Contribution of individual components to overall antioxidant activity and comparison with traditional antioxidant assays. J. Funct. Foods 2016, 24, 579–594. [Google Scholar] [CrossRef]
- Cuoco, G.; Mathe, C.; Vieillescazes, C. Liquid chromatographic analysis of flavonol compounds in green fruits of three Rhamnus species used in Stil de grain. Microchem. J. 2014, 115, 130–137. [Google Scholar] [CrossRef]
- Aguirre-Hernandez, E.; Gonzalez-Trujano, M.E.; Martinez, A.L.; Moreno, J.; Kite, G.; Terrazas, T.; Soto-Hernandez, M. HPLC/MS analysis and anxiolytic-like effect of quercetin and kaempferol flavonoids from Tilia americana var. mexicana. J. Ethnopharmacol. 2010, 127, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-L.; Cui, M.; He, Y.; Yu, H.-L.; Guo, D.-A. Chemical fingerprint and metabolic fingerprint analysis of Danshen injection by HPLC–UV and HPLC–MS methods. J. Pharmaceut. Biomed. 2005, 36, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Harnly, J.M. LC-MS profiling and quantification of food phenolic components using a standard analytical approach for all plants. In Food Science and Technology: New Research; Greco, L.V., Bruno, M.N., Eds.; Nova Publisher: New York, NY, USA, 2008. [Google Scholar]
- Keinänen, M.; Julkunen-Tiitto, R. High-performance liquid chromatographic determination of flavonoids in Betula pendula and Betula pubescens leaves. J. Chromatogr. A 1998, 793, 370–377. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, H.; Zhao, X.; Lv, Q.; Sun, C.; Li, X.; Chen, K. Effects of flavonoids-rich Chinese bayberry (Myrica rubra Sieb. et Zucc.) pulp extracts on glucose consumption in human HepG2 cells. J. Funct. Foods 2015, 14, 144–153. [Google Scholar] [CrossRef]
- Ncube, E.N.; Mhlongo, M.I.; Piater, L.A.; Steenkamp, P.A.; Dubery, I.A.; Madala, N.E. Analyses of chlorogenic acids and related cinnamic acid derivatives from Nicotiana tabacum tissues with the aid of UPLC-QTOF-MS/MS based on the in-source collision-induced dissociation method. Chem. Cent. J. 2014, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.Q.; Chen, Y.; Liang, X.T. Structural chemistry and biological activities of natural products from Chinese herbal medicines—Part II. Res. Commun. Mol. Path. 2000, 108, 393–436. [Google Scholar]
- Wen, L.; You, L.; Yang, X.; Yang, J.; Chen, F.; Jiang, Y.; Yang, B. Identification of phenolics in litchi and evaluation of anticancer cell proliferation activity and intracellular antioxidant activity. Free Radic. Biol. Med. 2015, 84, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.H.; Gao, L.F.; Gao, W.; Yuan, Y.S.; Gao, C.C.; Cao, L.G.; Hu, Z.Z.; Guo, J.Q.; Zhang, Y.X. Weight-reducing effect of Acer truncatum Bunge may be related to the inhibition of fatty acid synthase. Nat. Prod. Res. 2011, 25, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.H.; Gao, C.C.; Ma, X.F.; Bai, X.Y.; Zhang, Y.X. The isolation of 1,2,3,4,6-penta-O-galloyl-beta-D-glucose from Acer truncatum Bunge by high-speed counter-current chromatography. J. Chromatogr. B 2007, 850, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Sumczynski, D.; Bubelova, Z.; Sneyd, J.; Erb-Weber, S.; Mlcek, J. Total phenolics, flavonoids, antioxidant activity, crude fibre and digestibility in non-traditional wheat flakes and muesli. Food Chem. 2015, 174, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Alañón, M.E.; Castro-Vázquez, L.; Díaz-Maroto, M.C.; Gordon, M.H.; Pérez-Coello, M.S. A study of the antioxidant capacity of oak wood used in wine ageing and the correlation with polyphenol composition. Food Chem. 2011, 128, 997–1002. [Google Scholar] [CrossRef]
- Sample Availability: Not available.



| Run | Factors | Total Phenols (mg/100 g) | DPPH (μmol/100 g) | DPPH Activity/Total Phenols (μmol/mg) | |||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Experimental (Predicted) Values | |||
| C (%) | R (g/L) | P (W) | T (°C) | ||||
| 1 | 70(0) | 20(0) | 240(0) | 50(0) | 7467.83 (7449.86) | 71,695.05 (72,589.38) | 9.60 |
| 2 | 60(−1) | 15(−1) | 270(1) | 60(1) | 7591.30 (7534.35) | 70,380.34 (70,880.44) | 9.27 |
| 3 | 70(0) | 20(0) | 240(0) | 50(0) | 7417.39 (7449.86) | 73,356.78 (72,589.38) | 9.89 |
| 4 | 80(1) | 25(1) | 270(1) | 40(−1) | 6622.83 (6632.18) | 57,161.41 (56,161.13) | 8.63 |
| 5 | 80(1) | 25(1) | 210(−1) | 60(1) | 6840.22 (6732.65) | 51,733.24 (52,828.09) | 7.56 |
| 6 | 80(1) | 15(−1) | 270(1) | 60(1) | 7005.87 (6927.40) | 66,300.47 (65,584.34) | 9.46 |
| 7 | 60((−1) | 15(−1) | 210(−1) | 40(−1) | 7281.30 (7204.18) | 64,060.73 (63,443.39) | 8.80 |
| 8 | 60(−1) | 25(1) | 270(1) | 40(−1) | 7180.98 (7062.72) | 60,133.24 (61,057.29) | 8.37 |
| 9 | 70(0) | 20(0) | 240(0) | 70(2) | 7384.35 (7425.71) | 63,223.73 (62,238.86) | 8.56 |
| 10 | 60(−1) | 15(−1) | 270(1) | 40(−1) | 7158.91 (7192.11) | 60,282.07 (59,038.22) | 8.42 |
| 11 | 60(−1) | 25(1) | 210(−1) | 40(−1) | 7168.48 (7172.58) | 68,041.95 (68,609.08) | 9.49 |
| 12 | 70(0) | 20(0) | 240(0) | 30(−2) | 7037.83 (7125.72) | 56,420.30 (57,524.26) | 8.02 |
| 13 | 70(0) | 20(0) | 240(0) | 50(0) | 7256.52 (7449.86) | 70,844.41 (72,589.38) | 9.76 |
| 14 | 50(−2) | 20(0) | 240(0) | 50(0) | 6776.52 (6846.72) | 57,567.32 (56,833.36) | 8.50 |
| 15 | 80(1) | 15(−1) | 210(−1) | 40(−1) | 6609.78 (6505.13) | 58,025.57 (56,190.23) | 8.78 |
| 16 | 80(1) | 25(1) | 210(−1) | 40(−1) | 6772.83 (6774.90) | 60,425.89 (59,955.71) | 8.92 |
| 17 | 90(2) | 20(0) | 240(0) | 50(0) | 5783.04 (5842.09) | 42,030.84 (42,883.89) | 7.27 |
| 18 | 70(0) | 10(−2) | 240(0) | 50(0) | 7026.96 (7141.37) | 66,619.14 (67,862.93) | 9.48 |
| 19 | 70(0) | 20(0) | 300(2) | 50(0) | 7106.09 (7147.63) | 69,835.50 (68,702.84) | 9.83 |
| 20 | 60(−1) | 25(1) | 270(1) | 60(1) | 7148.64 (7178.92) | 62,292.05 (63,978.39) | 8.71 |
| 21 | 80(1) | 15(−1) | 210(−1) | 60(1) | 6625.54 (6688.92) | 58,877.87 (57,983.73) | 8.89 |
| 22 | 70(0) | 20(0) | 180(−2) | 50(0) | 6931.30 (7019.01) | 67,402.26 (68,654.02) | 9.72 |
| 23 | 70(0) | 20(0) | 240(0) | 50(0) | 7519.13 (7449.86) | 72,961.25 (72,589.38) | 9.70 |
| 24 | 70(0) | 20(0) | 240(0) | 50(0) | 7587.83 (7449.86) | 72,829.01 (72,589.38) | 9.60 |
| 25 | 70(0) | 20(0) | 240(0) | 50(0) | 7450.43 (7449.86) | 73,849.78 (72,589.38) | 9.91 |
| 26 | 80(1) | 25(1) | 270(1) | 60(1) | 6851.09 (6873.33) | 56,634.84 (57,282.08) | 8.27 |
| 27 | 70(0) | 30(2) | 240(0) | 50(0) | 7040.87 (7055.71) | 65,851.05 (64,726.36) | 9.35 |
| 28 | 80(1) | 15(−1) | 270(1) | 40(−1) | 6513.91 (6460.19) | 54,193.49 (55,542.27) | 8.32 |
| 29 | 60(−1) | 25(1) | 210(−1) | 60(1) | 7006.52 (7005.36) | 64,600.48 (63,281.61) | 9.22 |
| 30 | 60(−1) | 15(−1) | 210(−1) | 60(1) | 7346.74 (7263.01) | 66,185.78 (67,037.04) | 9.01 |
| Source | Coefficient Estimate | Sum of Squares | Degree of Freedom | Standard Error | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Y1 | Model | 7449.86 | 4.16 × 106 | 14 | 43.66 | 2.97 × 105 | 25.95 | <0.0001 ** |
| X1 | −251.16 | 1.51 × 106 | 1 | 21.83 | 1.51 × 106 | 132.36 | <0.0001 ** | |
| X2 | −21.42 | 1.10 × 104 | 1 | 21.83 | 1.10 × 104 | 0.96 | 0.3422 | |
| X3 | 32.15 | 2.48 × 104 | 1 | 21.83 | 2.48 × 104 | 2.17 | 0.1614 | |
| X4 | 75 | 1.35 × 105 | 1 | 21.83 | 1.35 × 105 | 11.8 | 0.0037 ** | |
| X1X2 | 75.34 | 9.08 × 104 | 1 | 26.74 | 9.08 × 104 | 7.94 | 0.0130 * | |
| X1X3 | −8.22 | 1.08 × 103 | 1 | 26.74 | 1.08 × 103 | 0.09 | 0.7628 | |
| X1X4 | 31.24 | 1.56 × 104 | 1 | 26.74 | 1.56 × 104 | 1.37 | 0.2609 | |
| X2X3 | −24.45 | 9.56 × 103 | 1 | 26.74 | 9.56 × 103 | 0.84 | 0.375 | |
| X2X4 | −56.51 | 5.11 × 104 | 1 | 26.74 | 5.11 × 104 | 4.47 | 0.0517 | |
| X3X4 | 70.85 | 8.03 × 104 | 1 | 26.74 | 8.03 × 104 | 7.02 | 0.0182 * | |
| X12 | −276.36 | 2.09 × 106 | 1 | 20.42 | 2.09 × 106 | 183.16 | <0.0001 ** | |
| X22 | −87.83 | 2.12 × 105 | 1 | 20.42 | 2.12 × 105 | 18.5 | 0.0006 ** | |
| X32 | −91.63 | 2.30 × 105 | 1 | 20.42 | 2.30 × 105 | 20.14 | 0.0004 ** | |
| X42 | −43.54 | 5.20 × 104 | 1 | 20.42 | 5.20 × 104 | 4.55 | 0.05 | |
| Residual | 1.72 × 105 | 15 | 1.14 × 104 | |||||
| Lack of Fit | 1.09 × 105 | 10 | 1.09 × 104 | 0.87 | 0.6032 | |||
| Pure Error | 6.26 × 104 | 5 | 1.25 × 104 | |||||
| Cor Total | 4.33 × 106 | 29 | ||||||
| R2 | 0.9604 | |||||||
| Adj R2 | 0.9233 | |||||||
| Pred R2 | 0.8341 | |||||||
| Adeq Precision | 22.3775 | |||||||
| C.V. % | 1.5169 | |||||||
| r | 0.98 | |||||||
| Y2 | Model | 72,589.38 | 1.57 × 109 | 14 | 608.6 | 1.12 × 108 | 50.34 | <0.0001 ** |
| X1 | −3487.37 | 2.92 × 108 | 1 | 304.3 | 2.92 × 108 | 131.34 | <0.0001 ** | |
| X2 | −784.14 | 1.48 × 107 | 1 | 304.3 | 1.48 × 107 | 6.64 | <0.0001 ** | |
| X3 | 12.2 | 3.57 × 103 | 1 | 304.3 | 3.57 × 103 | 0 | 0.0210 * | |
| X4 | 1178.65 | 3.33 × 107 | 1 | 304.3 | 3.33 × 107 | 15 | 0.9685 | |
| X1X2 | −350.05 | 1.96 × 106 | 1 | 372.69 | 1.96 × 106 | 0.88 | 0.0015 ** | |
| X1X3 | 939.3 | 1.41 × 107 | 1 | 372.69 | 1.41 × 107 | 6.35 | 0.3625 | |
| X1X4 | −450.04 | 3.24 × 106 | 1 | 372.69 | 3.24 × 106 | 1.46 | 0.0235 * | |
| X2X3 | −786.65 | 9.90 × 106 | 1 | 372.69 | 9.90 × 106 | 4.46 | 0.2459 | |
| X2X4 | −2230.28 | 7.96 × 107 | 1 | 372.69 | 7.96 × 107 | 35.81 | 0.052 | |
| X3X4 | 2062.14 | 6.80 × 107 | 1 | 372.69 | 6.80 × 107 | 30.62 | <0.0001 ** | |
| X12 | −5682.69 | 8.86 × 108 | 1 | 284.65 | 8.86 × 108 | 398.56 | <0.0001 ** | |
| X22 | −1573.68 | 6.79 × 107 | 1 | 284.65 | 6.79 × 107 | 30.56 | <0.0001 ** | |
| X32 | −977.74 | 2.62 × 107 | 1 | 284.65 | 2.62 × 107 | 11.8 | <0.0001 ** | |
| X42 | −3176.95 | 2.77 × 108 | 1 | 284.65 | 2.77 × 108 | 124.57 | 0.0037 ** | |
| Residual | 3.33 × 107 | 15 | 2.22 × 106 | <0.0001 ** | ||||
| Lack of Fit | 2.71 × 107 | 10 | 2.71 × 106 | 2.18 | 0.2015 | |||
| Pure Error | 6.22 × 106 | 5 | 1.24 × 106 | 0.2015 | ||||
| Cor Total | 1.60 × 109 | 29 | ||||||
| R2 | 0.9792 | |||||||
| Adj R2 | 0.9597 | |||||||
| Pred R2 | 0.8968 | |||||||
| Adeq Precision | 28.1801 | |||||||
| C.V. % | 2.3491 | |||||||
| r | 0.9898 |
| Peak | Rt (min) | [M − H]− (m/z) | Error (ppm) | Formula | MS/MS Fragments m/z (% Base Peak) | Proposed Compound |
|---|---|---|---|---|---|---|
| 1 | 2.35 | 191.0564 | 4.2 | C7H11O6 | 127.0405 (3.7), 111.0453 (1.2) | Quinic acid |
| 2 | 4.03 | 343.0654 | −3.2 | C14H15O10 | 191.0559 (28.0), 169.0139 (100.0), 125.0243 (73.3) | Theogallin |
| 3 | 4.29 | 169.0143 | 3.5 | C7H5O5 | 125.0244 (100), 169.0139 (74.1) | Gallic acid |
| 4 | 6.91 | 353.0864 | −2.5 | C16H17O9 | 191.0552 (100.0), 179.0348 (53.0), 135.0449 (69.3) | 3-O-Caffeoylquinic acid |
| 5 | 7.85 | 353.0862 | −3.1 | C16H17O9 | 191.0550 (100), 179.0339 (54.7), 135.0444 (68.0) | 5-O-Caffeoylquinic acid |
| 6 | 11.05 | 285.0612 | 0.7 | C12H13O8 | 153.0177 (25.6), 109.0272 (17.5) | Uralenneoside |
| 7 | 12.64 | 337.0918 | −1.5 | C16H17O8 | 191.0550 (47.6), 163.0393 (100.0), 119.0494 (65.8) | cis-4-p-Coumaroylquinic acid |
| 8 | 12.93 | 337.0923 | 0 | C16H17O8 | 191.0558 (19.4), 163.0392 (100.0), 119.0498 (54.1) | cis-5-p-Coumaroylquinic acid |
| 9 | 14.4 | 183.0323 | 16.4 | C8H7O5 | 183.0300 (31.4), 124.0194 (100.0) | 4-O-Methyl-gallate |
| 10 | 16.08 | 755.2031 | −0.5 | C33H39O20 | 609.1443 (54.7), 463.2144 (11.2), 301.0345 (21.1) | Quercetin-3-O-rhamninoside |
| 11 | 16.39 | 289.0717 | 1.7 | C15H13O6 | 245.0822 (32.9), 211.0291 (7.7) | (+)-Catechin |
| 12 | 16.91 | 863.1819 | −0.5 | C45H35O18 | 289.0710 (78.7) | cinnamtannin B1 |
| 13 | 18.65 | 479.0822 | −0.8 | C21H19O13 | 316.0219 (100.0), 287.0192 (16.2), 271.0237 (30.5) | Myricetin-O-hexoside I |
| 14 | 19.01 | 479.0828 | 0.4 | C21H19O13 | 316.0221 (100.0), 271.0249 (28.5) | Myricetin-O-hexoside II |
| 15 | 19.38 | 593.1513 | 1.2 | C27H29O15 | 593.1505 (100.0), 447.0915 (53.1), 301.0343 (61.8) | Quercetin-3,7-O-α-l-dirhamnopyranoside |
| 16 | 21.47 | 787.1001 | 0.9 | C34H27O22 | 615.0979 (18.8), 465.0670 (15.3), 169.0137 (22.7) | 1,2,3,6-Tetrakis-O-galloyl-β-D-glucose |
| 17 | 21.95 | 449.0727 | 1.6 | C20H17O12 | 316.0228 (100.0), 271.0247 (37.8) | Myricetin-arabinoside/xylopyranoside Isomer |
| 18 | 23.1 | 463.0878 | 0.2 | C21H19O12 | 316.0218 (100.0), 287.0194 (18.9) | Myricitrin |
| 19 | 24.15 | 463.0877 | 0 | C21H19O12 | 300.0267 (100.0), 255.0293 (20.9) | Quercetin-3-O-galactoside |
| 20 | 25.09 | 463.0884 | 1.5 | C21H19O12 | 300.0273 (100.0), 255.0297 (22.6) | Quercetin-3-O-glucoside (isoquercetin) |
| 21 | 26.51 | 939.1124 | 2.1 | C41H31O26 | 769.0892 (57.3), 617.0776 (20.1), 447.0559 (9.6), 169.0140 (94.8) | Pentagalloyl glucose isomer |
| 22 | 28.23 | 939.1109 | 0.5 | C41H31O26 | 769.0883 (51.5), 617.0775 (19.2), 447.0550 (9.1), 169.0140 (51.2) | Pentagalloyl glucose isomer |
| 23 | 30.12 | 433.0768 | −0.7 | C20H17O11 | 300.0271 (100.0), 255.0286 (26.8), 243.0291 (10.4) | Quercetin-3-O-arabinopyranoside |
| 24 | 33.32 | 433.0767 | −0.9 | C20H17O11 | 300.0266 (100.0), 271.0236 (59.7), 255.0297 (35.1) | Quercetin 3-O-arabinofuranoside |
| 25 | 34.94 | 447.0927 | 0 | C21H19O11 | 300.0270 (100.0), 271.0249 (51.2), 255.0295 (27.3) | Quercetin 3-O-rhamnoside |
| 26 | 56.11 | 1091.12 | −1 | C48H35O30 | 939.1072 (60.0), 769.0875 (22.8), 169.0133 (100.0) | Hexagalloyl glucose |
| 27 | 60.3 | 431.0974 | −0.9 | C21H19O10 | 285.0387 (100.0), 255.0288 (59.4), 227.0336 (34.9) | Kaempferol-3-O-α-l-rhamnoside |
| 28 | 69.26 | 609.1234 | −1.6 | C30H25O14 | 463.0871 (68.8), 300.0274 (95.3) | Quercetin-3-O-rutinoside |
| 29 | 71.72 | 301.0354 | 2 | C15H9O7 | 301.0344 (45.7), 243.0662 (100.0) | Quercetin |
| Independent Variables | Independent Levels | ||||
|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | |
| Solvent concentration, X1 (%) | 50 | 60 | 70 | 80 | 90 |
| Material-to-liquid ratio, X2 (g/mL) | 1:5 | 1:15 | 1:20 | 1:25 | 1:30 |
| Extraction temperature, X3 (°C) | 30 | 40 | 50 | 60 | 70 |
| Sonication power, X4 (W) | 180 | 210 | 240 | 270 | 300 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Yin, P.; Fan, H.; Xue, Q.; Li, K.; Li, X.; Sun, L.; Liu, Y. Response Surface Methodology Optimization of Ultrasonic-Assisted Extraction of Acer Truncatum Leaves for Maximal Phenolic Yield and Antioxidant Activity. Molecules 2017, 22, 232. https://doi.org/10.3390/molecules22020232
Yang L, Yin P, Fan H, Xue Q, Li K, Li X, Sun L, Liu Y. Response Surface Methodology Optimization of Ultrasonic-Assisted Extraction of Acer Truncatum Leaves for Maximal Phenolic Yield and Antioxidant Activity. Molecules. 2017; 22(2):232. https://doi.org/10.3390/molecules22020232
Chicago/Turabian StyleYang, Lingguang, Peipei Yin, Hang Fan, Qiang Xue, Ke Li, Xiang Li, Liwei Sun, and Yujun Liu. 2017. "Response Surface Methodology Optimization of Ultrasonic-Assisted Extraction of Acer Truncatum Leaves for Maximal Phenolic Yield and Antioxidant Activity" Molecules 22, no. 2: 232. https://doi.org/10.3390/molecules22020232
APA StyleYang, L., Yin, P., Fan, H., Xue, Q., Li, K., Li, X., Sun, L., & Liu, Y. (2017). Response Surface Methodology Optimization of Ultrasonic-Assisted Extraction of Acer Truncatum Leaves for Maximal Phenolic Yield and Antioxidant Activity. Molecules, 22(2), 232. https://doi.org/10.3390/molecules22020232