Design of Drug Delivery Systems Containing Artemisinin and Its Derivatives
Abstract
:1. Introduction
2. Artemisinin Derivatives
3. Pharmacokinetics of Artemisinin and Its Derivatives
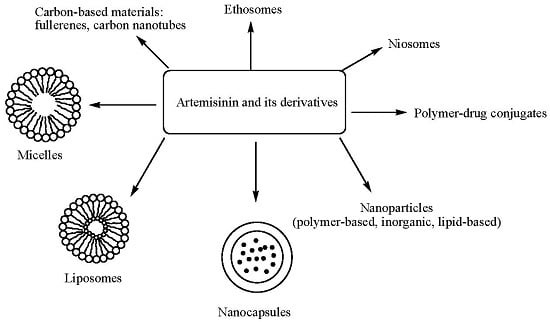
4. Delivery Systems Loaded with Artemisinin and Its Derivatives
4.1. Polymer-Drug Conjugates
4.2. Micelles
4.3. Liposomes
4.4. Nanocapsules
4.5. Niosomes
4.6. Ethosomes
4.7. Carbon-Based Materials
4.8. Nanoparticles
4.8.1. Lipid Based Nanoparticles
4.8.2. Polymer-Based and Inorganic-Based Nanoparticles
4.8.2.1. Polymer-Based Nanoparticles
4.8.2.2. Inorganic-Based Nanoparticles
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Whirl-Carrillo, M.; McDonagh, E.M.; Hebert, J.M.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Altman, R.B.; Klein, T.E. Pharmacogenomics Knowledge for Personalized Medicine. Clin. Pharmacol. Ther. 2012, 92, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Blazquez, A.G.; Fernandez-Dolon, M.; Sanchez-Vicente, L.; Maestre, A.D.; Gomez-San Miguel, A.B.; Alvarez, M.; Serrano, M.A.; Jansen, H.; Efferth, T.; Marin, J.J.; et al. Novel artemisinin derivatives with potential usefulness against liver/colon cancer and viral hepatitis. Bioorg. Med. Chem. 2013, 21, 4432–4441. [Google Scholar] [CrossRef] [PubMed]
- Artemisinin Effects, Health Benefits and Uses. Available online: http://nootriment.com/artemisinin/ (accessed on 12 December 2016).
- Efferth, T.; Romero, M.R.; Wolf, D.G.; Stamminger, T.; Marin, J.J.; Marschall, M. The antiviral activities of artemisinin and artesunate. Clin. Infect. Dis. 2008, 47, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Loo, C.S.; Lam, N.S.; Yu, D.; Su, X.Z.; Lu, F. Artemisinin and its derivatives in treating protozoan infections beyond malaria. Pharmacol. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Mutabingwa, T.K. Artemisinin-based combination therapies (ACTs): Best hope for malaria treatment but inaccessible to the needy. Acta Trop. 2005, 95, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Okell, L.C.; Cairns, M.; Griffin, J.T.; Ferguson, N.M.; Tarning, J.; Jagoe, G.; Hugo, P.; Baker, M.; D’Alessandro, U.; Bousema, T.; et al. Contrasting benefits of different artemisinin combination therapies as first-line malaria treatments using model-based cost-effectiveness analysis. Nat. Commun. 2014, 5, 5606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medhi, B.; Patyar, S.; Rao, R.S.; Byrav, D.S.P.; Prakash, A. Pharmacokinetic and toxicological profile of artemisinin compounds: an update. Pharmacol. 2009, 84, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wicht, K.J.; Shaban, E.; Ngoc, T.A.; Wang, M.Q.; Hayashi, I.; Hossain, M.I.; Takemasa, Y.; Kaiser, M.; El Sayed, I.E.; et al. Synthesis and evaluation of artesunate-indoloquinoline hybrids as antimalarial drug candidates. MedChemComm 2014, 5, 927–931. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, F.; Zhang, Z.; Chen, Y.; Wang, J. Synthesis and biological evaluation of a novel artesunate-podophyllotoxin conjugate as anticancer agent. Bioorg. Med. Chem. Lett. 2016, 26, 38–42. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Ouyang, X.; Huang, X.; Hu, W.; Dai, W.; Tian, X.; Pan, Y.; Huang, S.; Wang, H. Synthesis of derivatives of artesunate α-aminophosphonate and their antimicrobial activities. Lett. Drug Des. Discov. 2015, 12, 408–416. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; de Kiff, A.; Neudörfl, J.M.; Sillner, S. Singlet oxygen addition to cyclo-1,3-hexadienes from natural sources and from organocatalytic enal dimerization. ARKIVOC 2015, 3, 101–110. [Google Scholar]
- Capela, R.; Cabal, G.G.; Rosenthal, P.J.; Gut, J.; Mota, M.M.; Moreira, R.; Lopes, F.; Prudêncio, M. Design and evaluation of primaquine-artemisinin hybrids as a multistage antimalarial strategy. Antimicrob. Agents Chemother. 2011, 55, 4698–4706. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, Z.S.; Zhang, H.; Cao, B.J.; Wang, F.D.; Zhang, Y.; Shi, Y.L.; Yang, J.D.; Wu, B.A. Artemisinin derivatives bearing Mannich base group: Synthesis and antimalarial activity. Bioorg. Med. Chem. 2003, 11, 4363–4368. [Google Scholar] [CrossRef]
- Chand, H.R.; Bhattacharya, A.K. Diastereoselective Synthesis of β-Ether Derivatives of Artemisinin, an Antimalarial Drug: The Effect of Nitrile on Stereoselectivity. Asian J. Org. Chem. 2015, 5, 201–206. [Google Scholar] [CrossRef]
- Opsenica, D.M.; Šolaja, B.A. Artemisinins and synthetic peroxides as highly efficient antimalarials. Maced. J. Chem. Chem. Eng. 2012, 31, 137–182. [Google Scholar]
- Walsh, J.J.; Coughlan, D.; Heneghan, N.; Gaynor, C.; Bell, A. A novel artemisinin-quinine hybrid with potent antimalarial activity. Bioorg. Med. Chem. Lett. 2007, 17, 3599–3602. [Google Scholar] [CrossRef] [PubMed]
- Joubert, J.P.; Smit, F.J.; du Plessis, L.; Smith, P.J.; N’Da, D.D. Synthesis and in vitro biological evaluation of aminoacridines and artemisinin-acridine hybrids. Eur. J. Pharm. Sci. 2014, 56, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.K.; Biswas, S.; Gunjan, S.; Chauhan, B.S.; Singh, S.K.; Srivastava, K.; Singh, S.; Batra, S.; Tripathi, R. Pyrrolidine-Acridine hybrid in Artemisinin-based combination: A pharmacodynamic study. Parasitology 2016, 143, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Guiguemde, A.W.; Bentura-Marciano, A.; Clark, J.; Haynes, R.K.; Chan, W.C.; Wong, H.N.; Hunt, N.H.; Guy, R.K.; Golenser, J. Synthesis of artemiside and its effects in combination with conventional drugs against severe murine malaria. Antimicrob. Agents Chemother. 2012, 56, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Xu, J.; Zhang, H.; Li, X. Synthesis and Anti-tumor Effect of Artemisone Derivatives. Chin. J. Org. Chem. 2015, 35, 1097. [Google Scholar] [CrossRef]
- Xu, J.; Wei, M.; Li, G.; Li, X. Synthesis and Anti-tumor Activities of Novel Artemisone-piperazine-sulfonamide Derivatives. Chem. J. Chin. 2015, 36, 919–926. [Google Scholar]
- Soomro, S.; Langenberg, T.; Mahringer, A.; Konkimalla, V.B.; Horwedel, C.; Holenya, P.; Brand, A.; Cetin, C.; Fricker, G.; Dewerchin, M.; et al. Design of novel artemisinin-like derivatives with cytotoxic and anti-angiogenic properties. J. Cell. Mol. Med. 2011, 15, 1122–1135. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.C.; Deng, T.; Fan, M.L.; Lv, W.B.; Liu, J.H.; Yu, B.Y. Synthesis and in vitro antitumor evaluation of dihydroartemisinin-cinnamic acid ester derivatives. Eur. J. Med. Chem. 2016, 107, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.A.; Duparc, S.; Borghini-Fuhrer, I.; Jung, D.; Shin, C.S.; Fleckenstein, L. Review of the clinical pharmacokinetics of artesunate and its active metabolite dihydroartemisinin following intravenous, intramuscular, oral or rectal administration. Malar. J. 2011, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for the Treatment of Malaria; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Saifi, M.A.; Beg, T.; Harrath, A.H.; Altayalan, F.S.; Al Quraishy, S. Antimalarial drugs: Mode of action and status of resistance. Afr. J. Pharm. Pharmacol. 2013, 7, 148–156. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Little, B.; Meshnick, S.R. Alkylation of proteins by artemisinin. Effects of heme, pH, and drug structure. Biochem. Pharmacol. 1994, 48, 569–573. [Google Scholar] [CrossRef]
- Das, A.K. Anticancer Effect of AntiMalarial Artemisinin Compounds. Ann. Med. Health Sci. Res. 2015, 5, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Olliaro, P.L.; Haynes, R.K.; Meunier, B.; Yuthavong, Y. Possible modes of action of the artemisinin-type compounds. Trends Parasitol. 2001, 17, 122–126. [Google Scholar] [CrossRef]
- Lu, J.J.; Meng, L.H.; Shankavaram, U.T.; Zhu, C.H.; Tong, L.J.; Chen, G.; Lin, L.P.; Weinstein, J.N.; Ding, J. Dihydroartemisinin accelerates c-MYC oncoprotein degradation and induces apoptosis in c-MYC-overexpressing tumor cells. Biochem. Pharmacol. 2010, 80, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.J.; Chen, S.M.; Zhang, X.W.; Ding, J.M.; Meng, L.H. The anti-cancer activity of dihydroartemisinin is associated with induction of iron-dependent endoplasmic reticulum stress in colorectal carcinoma HCT116 cells. Investig. New Drugs 2011, 29, 1276–1283. [Google Scholar] [CrossRef] [PubMed]
- Mercer, A.E.; Copple, I.M.; Maggs, J.L.; O’Neill, P.M.; Park, B.K. The role of heme and the mitochondrion in the chemical and molecular mechanisms of mammalian cell death induced by the artemisinin antimalarials. J. Biol. Chem. 2011, 286, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Qian, R.; Li, Z.; Yu, J.; Ma, D. The Immunologic and Antiviral Effect of Qinghaosu. J. Tradit. Chin. Med. 1982, 2, 271–276. [Google Scholar] [PubMed]
- Kaptein, S.J.; Efferth, T.; Leis, M.; Rechter, S.; Auerochs, S.; Kalmer, M.; Bruggeman, C.A.; Vink, C.; Stamminger, T.; Marschall, M. The anti-malaria drug artesunate inhibits replication of cytomegalovirus in vitro and in vivo. Antivir. Res. 2006, 69, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, J.; Canal, F.; Lucas, R.; Vicent, M.J. Polymer-drug conjugates for novel molecular targets. Nanomedicine 2010, 5, 915–935. [Google Scholar] [CrossRef] [PubMed]
- Vasey, P.A.; Kaye, S.B.; Morrison, R.; Twelves, C.; Wilson, P.; Duncan, R.; Thomson, A.H.; Murray, L.S.; Hilditch, T.E.; Murray, T.; et al. Phase I clinical and pharmacokinetic study of PK1 [N-(2-hydroxypropyl) methacrylamide copolymer doxorubicin]: First member of a new class of chemotherapeutic agents-drug-polymer conjugates. Clin. Cancer Res. 1999, 5, 83–94. [Google Scholar] [PubMed]
- Ringsdorf, H. Structure and properties of pharmacologically active polymers. J. Polym. Sci. C Polym. Symp. 1975, 51, 135–153. [Google Scholar] [CrossRef]
- Terwogt, J.M.; ten Bokkel Huinink, W.W.; Schellens, J.H.; Schot, M.; Mandjes, I.A.; Zurlo, M.G.; Rocchetti, M.; Rosing, H.; Koopman, F.J.; Beijnen, J.H. Phase I clinical and pharmacokinetic study of PNU166945, a novel water-soluble polymer-conjugated prodrug of paclitaxel. Anticancer Drugs 2001, 12, 315–323. [Google Scholar] [CrossRef]
- Liu, K.; Dai, L.; Li, C.; Liu, J.; Wang, L.; Lei, J. Self-assembled targeted nanoparticles based on transferrin-modified eight-arm-polyethylene glycol-dihydroartemisinin conjugate. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Li, H.; Gu, J.; Guo, T.; Yang, S.; Guo, Z.; Zhang, X.; Zhu, W.; Zhang, J. Ternary system of dihydroartemisinin with hydroxypropyl-β-cyclodextrin and lecithin: Simultaneous enhancement of drug solubility and stability in aqueous solutions. J. Pharm. Biomed. Anal. 2013, 83, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Wang, L.; Deng, L.; Liu, J.; Lei, J.; Li, D.; He, J. Novel multiarm polyethylene glycol-dihydroartemisinin conjugates enhancing therapeutic efficacy in non-small-cell lung cancer. Sci. Rep. 2014, 4, 5871. [Google Scholar] [CrossRef] [PubMed]
- Yaméogo, J.B.; Gèze, A.; Choisnard, L.; Putaux, J.L.; Gansané, A.; Sirima, S.B.; Semdé, R.; Wouessidjewe, D. Self-assembled biotransesterified cyclodextrins as Artemisinin nanocarriers—I: Formulation, lyoavailability and in vitro antimalarial activity assessment. Eur. J. Pharm. Biopharm. 2012, 80, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.; Yang, B.; Chen, Y.J.; Liao, X.L.; Yang, X.M.; Qin, Q.X.; Yi, D. Synthesis of Water Soluble C-10-Phenoxy Artemisinin-Chitosan Conjugate. Asian J. Chem. 2013, 25, 4654–4656. [Google Scholar]
- Kumar, S.; Singh, R.K.; Murthy, R.S.; Bhardwaj, T.R. Synthesis and Evaluation of Substituted Poly(organophosphazenes) as a Novel Nanocarrier System for Combined Antimalarial Therapy of Primaquine and Dihydroartemisinin. Pharm. Res. 2015, 32, 2736–2752. [Google Scholar] [PubMed]
- Bhadra, D.; Bhadra, S.; Jain, N.K. Pegylated lysine based copolymeric dendritic micelles for solubilization and delivery of artemether. J. Pharm. Pharm. Sci. 2005, 8, 467–482. [Google Scholar] [PubMed]
- Jabbarzadegan, M.; Rajayi, H.; Mofazzal Jahromi, M.A.; Yeganeh, H.; Yousefi, M.; Muhammad Hassan, Z.; Majidi, J. Application of arteether-loaded polyurethane nanomicelles to induce immune response in breast cancer model. Artif. Cells Nanomed. Biotechnol. 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, Y.; Ma, J.; Zhang, H.; Zhang, H.; Wang, X.; Wang, J.; Zhang, X.; Zhang, Q. LyP-1 modification to enhance delivery of artemisinin or fluorescent probe loaded polymeric micelles to highly metastatic tumor and its lymphatics. Mol. Pharm. 2012, 9, 2646–2657. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.F.; Chen, S.F.; Wen, Z.Y.; Li, Q.; Chen, J.H. In vitro evaluation of efficacy of dihydroartemisinin-loaded methoxy poly (ethylene glycol)/poly (l-lactic acid) amphiphilic block copolymeric micelles. J. Appl. Polym. Sci. 2013, 128, 3084–3092. [Google Scholar] [CrossRef]
- Kim, S.; Park, K. Polymer Micelles for Drug Delivery. Chapter 19. Available online: http://kinam.com/Articles/PMicelles%20Papers/Kim,%20SW%2010%20Pol%20Micelle%20BookCh.pdf (accessed on 23 December 2016).
- Çağdaş, M.; Sezer, A.D.; Bucak, S. Liposomes as Potential Drug Carrier Systems for Drug Delivery. In Nanotechnology and Nanomaterials. Application of Nanotechnology in Drug Delivery; Sezer, A.D., Ed.; Intech: Rijeka, Croatia, 2014; pp. 1–50. [Google Scholar]
- Chen, H.J.; Huang, X.R.; Zhou, X.B.; Zheng, B.Y.; Huang, J.D. Potential sonodynamic anticancer activities of artemether and liposome-encapsulated artemether. Chem. Commun. 2015, 51, 4681–4684. [Google Scholar] [CrossRef] [PubMed]
- Neda, D.; Dariush, N.; Mohsen, C.; Hassan, E.S.; Mohammadreza, M.S.; Ali, F.; Akbarzadeh, A. Effect of artemisinin liposome and artemisinin liposome polyethyleneglycol on MCF-7 cell line. Int. J. Life Sci. Biotechnol. Pharm. Res. 2013, 2, 349–355. [Google Scholar]
- Isacchi, B.; Bergonzi, M.C.; Grazioso, M.; Righeschi, C.; Pietretti, A.; Severini, C.; Bilia, A.R. Artemisinin and artemisinin plus curcumin liposomal formulations: Enhanced antimalarial efficacy against Plasmodium berghei-infected mice. Eur. J. Pharm. Biopharm. 2012, 80, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Y.; Zhao, Y.; Sun, M.G.; Shi, J.F.; Ju, R.J.; Zhang, C.X.; Li, X.T.; Zhao, W.Y.; Mu, L.M.; Zeng, F.; et al. Multifunctional liposomes loaded with paclitaxel and artemether for treatment of invasive brain glioma. Biomaterials 2014, 35, 5591–5604. [Google Scholar] [CrossRef] [PubMed]
- Isacchi, B.; Arrigucci, S.; Marca, G.L.; Bergonzi, M.C.; Vannucchi, M.G.; Novelli, A.; Bilia, A.R. Conventional and long-circulating liposomes of artemisinin: Preparation, characterization, and pharmacokinetic profile in mice. J. Liposome Res. 2011, 21, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Gharib, A.; Faezizadeh, Z.; Mesbah-Namin, S.A.; Saravani, R. Preparation, characterization and in vitro efficacy of magnetic nanoliposomes containing the artemisinin and transferrin. DARU J. Pharm. Sci. 2014, 22, 1. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Shen, X.; Zhao, C.; Qin, X.; Liu, H.; Huang, L.; Qiu, Z.; Liu, Y. In vivo study of effects of artesunate nanoliposomes on human hepatocellular carcinoma xenografts in nude mice. Drug Deliv. 2013, 20, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Dadgar, N.; Alavi, S.E.; Esfahani, M.K.; Akbarzadeh, A. Study of toxicity effect of pegylated nanoliposomal artemisinin on breast cancer cell line. Ind. J. Clin. Biochem. 2013, 28, 410–412. [Google Scholar] [CrossRef] [PubMed]
- Righeschi, C.; Coronnello, M.; Mastrantoni, A.; Isacchi, B.; Bergonzi, M.C.; Mini, E.; Bilia, A.R. Strategy to provide a useful solution to effective delivery of dihydroartemisinin: Development, characterization and in vitro studies of liposomal formulations. Colloid Surf. B Biointerfaces 2014, 116, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lin, X.; Park, H.; Greever, R. Study of artemisinin nanocapsules as anticancer drug delivery systems. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Nguyen, T.C.; Poudel, B.K.; Nguyen, H.T.; Kim, J.O.; Yong, C.S.; Nguyen, C.N. Development and Evaluation of Artesunate-Loaded Chitosan-Coated Lipid Nanocapsule as a Potential Drug Delivery System Against Breast Cancer. AAPS PharmSciTech 2015, 16, 1307–1316. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Xu, K.; Xu, Y.; Luo, P.; Du, F.; Huang, J.; Lu, W.; Yu, J.; Liu, S.; Muir, B. Nanocapsules based on mPEGylated artesunate prodrug and its cytotoxicity. Colloids Surf. B Biointerfaces 2014, 115, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.-C.; Hong, Z.-G. Firstborn microcrystallization method to prepare nanocapsules containing artesunate. Int. J. Nanomed. 2010, 5, 483–486. [Google Scholar] [CrossRef]
- Dwivedi, A.; Mazumder, A.; Du Plessis, L.; Du Preez, J.L.; Haynes, R.K.; Du Plessis, J. In vitro anti-cancer effects of artemisone nano-vesicular formulations on melanoma cells. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 2041–2050. [Google Scholar] [CrossRef] [PubMed]
- Asgharkhani, E.; Najmafshar, A.; Chiani, M. Research Article Artemisinin (ART) Drug Delivery Using Mixed Non-ionic Surfactants and Evaluation of Their Efficiency in Different Cancer Cell Lines. Int. J. Drug Deliv. Technol. 2014, 4, 67–71. [Google Scholar]
- Shen, S.; Liu, S.Z.; Zhang, Y.S.; Du, M.B.; Liang, A.H.; Song, L.H.; Ye, Z.G. Compound antimalarial ethosomal cataplasm: Preparation, evaluation, and mechanism of penetration enhancement. Int. J. Nanomed. 2015, 10, 4239–4253. [Google Scholar] [CrossRef] [PubMed]
- Kothamasu, P.; Kanumur, H.; Ravur, N.; Maddu, C.; Parasuramrajam, R.; Thangavel, S. Nanocapsules: The weapons for novel drug delivery systems. Bioimpacts 2012, 2, 71–81. [Google Scholar] [PubMed]
- Effertha, T.; Romero, M.R.; Bilia, A.R.; Osman, A.G.; ElSohly, M.; Wink, M.; Bauer, R.; Ikhlas, K.H.; Bergonzi, M.C.; Marin, J.J.G. Expanding the Therapeutic Spectrum of Artemisinin: Activity Against Infectious Diseases Beyond Malaria and Novel Pharmaceutical Developments. World J. Tradit. Chin. Med. 2016, 2, 1–23. [Google Scholar] [CrossRef]
- Nandure, H.P.; Puranik, P.; Giram, P.; Lone, V. Ethosome: A Novel Drug Carrier. Int. J. Pharm. Res. Allied Sci. 2013, 2, 18–30. [Google Scholar]
- Cha, C.; Shin, S.R.; Annabi, N.; Dokmeci, M.R.; Khademhosseini, A. Carbon-based nanomaterials: Multifunctional materials for biomedical engineering. ACS Nano 2013, 7, 2891–2897. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, B.; Majidi, N.; Noori, S.; Hassan, Z.M. Multiwalled carbon nanotubes effect on the bioavailability of artemisinin and its cytotoxity to cancerous cells. J. Nanopart. Res. 2011, 13, 6339–6346. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, Y.; Chen, Q.; Jiao, X.; Hou, L.; Zhu, X.; Zhang, Z. Enhancement of cytotoxicity of artemisinin toward cancer cells by transferrin-mediated carbon nanotubes nanoparticles. J. Drug Target. 2015, 23, 552–567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hou, L.; Jiao, X.; Ji, Y.; Zhu, X.; Zhang, Z. Transferrin-mediated fullerenes nanoparticles as Fe2+-dependent drug vehicles for synergistic anti-tumor efficacy. Biomaterials 2015, 37, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gallis, B.; Taya, M.; Wang, S.; Ho, R.J.; Sasaki, T. pH-responsive artemisinin derivatives and lipid nanoparticle formulations inhibit growth of breast cancer cells in vitro and induce down-regulation of HER family members. PLoS ONE 2013, 8, e59086. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, P.; Khatik, R.; Khandelwal, K.; Taneja, I.; Raju, K.S.; Paliwal, S.K.; Dwivedi, A.K.; Mishra, P.R. Pharmacokinetics study of arteether loaded solid lipid nanoparticles: An improved oral bioavailability in rats. Int. J. Pharm. 2014, 466, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qiao, H.; Liu, J.; Dong, H.; Shen, C.; Ni, J.; Shi, Y.; Xu, Y. Dihydroartemisinin loaded nanostructured lipid carriers (DHA-NLC): Evaluation of pharmacokinetics and tissue distribution after intravenous administration to rats. Die Pharm. Int. J. Pharm. Sci. 2010, 65, 670–678. [Google Scholar]
- Aditya, N.P.; Patankar, S.; Madhusudhan, B.; Murthy, R.S.; Souto, E.B. Arthemeter-loaded lipid nanoparticles produced by modified thin-film hydration: Pharmacokinetics, toxicological and in vivo anti-malarial activity. Eur. J. Pharm. Sci. 2010, 40, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Want, M.Y.; Islamuddin, M.; Chouhan, G.; Ozbak, H.A.; Hemeg, H.A.; Dasgupta, A.K.; Chattopadhyay, A.P.; Afrin, F. Therapeutic efficacy of artemisinin-loaded nanoparticles in experimental visceral leishmaniasis. Colloids Surf. B Biointerfaces 2015, 130, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.K.; Razdan, B.K.; Bajpai, M. Formulation and evaluation of nanoparticles containing artemisinin HCL. Int. J. Res. Dev. Pharm. Sci. 2014, 3, 925–934. [Google Scholar]
- Nguyen, H.T.; Tran, T.H.; Kim, J.O.; Yong, C.S.; Nguyen, C.N. Enhancing the in vitro anti-cancer efficacy of artesunate by loading into poly-d,l-lactide-co-glycolide (PLGA) nanoparticles. Arch. Pharm. Res. 2015, 38, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Chadha, R.; Gupta, S.; Pathak, N. Artesunate-loaded chitosan/lecithin nanoparticles: Preparation, characterization, and in vivo studies. Drug Dev. Ind. Pharm. 2012, 38, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Teong, B.; Chen, I.F.; Chang, S.J.; Gao, J.; Kuo, S.M. Enhanced apoptotic effects of dihydroartemisinin-aggregated gelatin and hyaluronan nanoparticles on human lung cancer cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.; Ibrahim, H.; Sabater, A.M.; Mazier, D.; Valentin, A.; Nepveu, F. Artemisinin nanoformulation suitable for intravenous injection: Preparation, characterization and antimalarial activities. Int. J. Pharm. 2015, 495, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Xu, A.; Ying, J.; Li, B.; Jin, Y. Biodegradable Core-Shell Copolymer-Phospholipid Nanoparticles for Combination Chemotherapy: An In Vitro Study. J. Biomed. Nanotechnol. 2015, 11, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, Y.; Yang, Y.; Yang, J.; Guo, X.; Zhang, J.; Pan, L.; Xia, G.; Chen, B. Effect of interaction of magnetic nanoparticles of Fe3O4 and artesunate on apoptosis of K562 cells. Int. J. Nanomed. 2011, 6, 1185. [Google Scholar]
- Chen, J.; Guo, Z.; Wang, H.B.; Zhou, J.J.; Zhang, W.J.; Chen, Q.W. Multifunctional mesoporous nanoparticles as pH-responsive Fe2+ reservoirs and artemisinin vehicles for synergistic inhibition of tumor growth. Biomaterials 2014, 35, 6498–6507. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Manoli, F.; Manet, I.; Daoud-Mahammed, S.; Agostoni, V.; Gref, R.; Monti, S. β-Cyclodextrin polymer nanoparticles as carriers for doxorubicin and artemisinin: A spectroscopic and photophysical study. Photochem. Photobiol. Sci. 2012, 11, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Kakran, M.; Sahoo, N.G.; Li, L.; Judeh, Z. Dissolution of artemisinin/polymer composite nanoparticles fabricated by evaporative precipitation of nanosuspension. J. Pharm. Pharmacol. 2010, 62, 413–421. [Google Scholar] [CrossRef] [PubMed]











| Artemisinin Hybrid Compounds | Biological Activity | In Vivo/In Vitro Outcome | Ref. |
|---|---|---|---|
| Artesunate-indoloquinoline hybrid 6 | Antimalarial | The hybrid compound exhibited decreased cytotoxicity and increased antimalarial activity compared to the individual compounds. A dose of 10 mg·kg−1 once a day for four consecutive days resulted in a significant reduction of parasitemia. | [9] |
| Artesunate-podophyllotoxin analogue 7 | Anticancer | The hybrid compound exhibited good cytotoxicity effects on cancer cell lines with reduced resistant factor. It induced D G2/M cell cycle arrest in multidrug resistance K562/ADR cells. | [10] |
| Artesunate α-aminophosphonate analogue 8 | Antimicrobial | The hybrid compound exhibited significant activity against Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Candida albicans microbials. | [11] |
| Artesunate-safranol analogue 9 | - | - | [12] |
| Primaquine-artemisinin analogue 10 | Antimalaria | The hybrids were more effective against the liver and blood stages of malarial parasites compared to the individual compounds. | [13] |
| Arteminisin derivatives containing Mannich base group | Antimalaria | The derivatives were very stable and effective against Plasmodium berghei in mice when compared to the free artesunic acid. | [14] |
| β-Ether derivatives of dihydroartemisinin | Antimalaria | - | [15] |
| Artemisinin derivatives 11 and 12 | Antimalaria | The derivatives exhibited good antimalarial activity. | [16] |
| Artemisinin-quinine hybrids 13 | Antimalaria | The hybrid compounds exhibited potent antimalarial activity against the 3D7 and drug-resistant FcB1 strains of Plasmodium falciparum in vitro than the individual drugs | [17] |
| Artemisinin-acridine hybrids | Antimalaria and anticancer | The hybrid exhibited seven-fold higher anti-gametocytocidal effect. The anticancer activity against the HeLa cells was between three to eight fold higher than the individual drugs. | [18] |
| Pyrrolidine-acridine-artemisinin hybrid | Antimalarial | The antimalarial activity of the hybrid was dose dependent resulting in haem bio-mineralization inhibition. It was effective for the treatment of multiple drug resistant with no sign of toxicity in vivo. | [19] |
| Artemisone and artemiside derivatives | Antimalarial | The derivatives increased drug concentrations in the combination therapy without reaching toxic levels and were active against Plasmodium falciparum. | [20] |
| Artemisone derivatives | Antitumor | In vitro activity of the derivatives on human hepatoma SMMC-7721 cell lines revealed that the derivatives inhibited proliferation of the liver cancer cells by inducing apoptosis. | [21,22] |
| Artemisinin derivatives | Anticancer | The derivatives were stable at room temperature, disrupting drug-resistance pathways when compared to artesunate. | [23,24] |
| Dihydroartemisinin-cinnamic acid hybrids 14 | Anticancer | In vitro antitumor activities of the hybrids against PC-3, SGC-7901, A549 and MDA-MB-435s cancer cell lines revealed that the hybrids were active against lung cancer. | [25] |
| Polymer | Artemisinin Derivatives | Drug Delivery System | Application | Advantages | Ref. |
|---|---|---|---|---|---|
| Polyethylene glycol | Dihydroartemisinin | Polymer-drug conjugate | Antitumor | Enhanced solubility, long circulating half-life and improved antitumor effect. | [40,42] |
| Hydroxypropyl-β-cyclodextrin | Dihydroartemisinin | Polymer-drug conjugate | Oral administration | Increased solubility and stability of dihydroartemisinin. | [41] |
| Cyclodextrin via vinyl-acyl fatty esters | Artemisinin | Polymer-drug conjugate | Antimalarial by injection. | Effective antimalarial effect against P. falciparum. | [43] |
| Chitosan | Artemisinin | Polymer-drug conjugate | - | Improved solubility of artemisinin than the free artemisinin. | [44] |
| Polyorgano-phosphazenes | Primaquine and dihydroartemisinin | Polymer-drug conjugate | Antimalarial | Effective for combination therapy against drug resistant strain of P. berghei infected mice with 100% antimalarial activity. | [45] |
| Methoxy-polyethylene glycol | Artemether | Micelles | Antimalarial | Enhanced drug stability with prolonged release of artemether. | [46] |
| Polyurethane | Arteether | Micelles | Anticancer | Significant inhibition of the growth of 4T1 cell line. | [47] |
| PEG-PCL | Artemisinin | Micelles | Antitumor | High cellular uptake and inhibition effects on cancer cell lines with good antitumor efficacy. | [48] |
| Methoxy poly(ethylene glycol)/poly(l-lactic acid) | Dihydroartemisinin | Micelles | Anticancer | Better solubilizing ability than dihydroartemisinin suspension. | [49] |
| Artemisinin Derivative | Drug Delivery System | Application | Advantage | Ref. |
|---|---|---|---|---|
| Artemether | Lipososmes | Anticancer | Enhanced sonodynamic anticancer activity. | [52] |
| Artemisinin | Liposomes | Anticancer | Enhanced cytotoxicity effects on MCF-7 cell lines. | [53] |
| Artemisinin | Liposomes | Antimalarial | Immediate and enhanced antimalarial activity. | [54] |
| Artemeter | Liposomes | Anticancer | Effective for invasive brain glioma. | [55] |
| Artemisinin | Liposomes | Antitumor and antiparasitic | Enhanced blood-circulation time and prolonged half-life. | [56] |
| Artemisinin | Magnetic liposomes | Anticancer | Thermosensitive with high antiproliferative activity. | [57,59] |
| Artesunate | Liposomes | Antitumor | Enhanced antitumor effects on human hepatoma HepG2 cells than the free artesunate | [58] |
| Dihydroartemisinin | Liposomes | Anticancer | Enhanced anticancer activity. | [60] |
| Artemisinin | Nanocapules | Anticancer | Prolonged release mechanism with enhanced bioavailability | [61,62,63,64] |
| Artemisone | Niosomes | Anticancer | Enhanced selective anticancer activity towards human melanoma A-375 cells with no toxicity towards the normal skin cells. | [65] |
| Artesunate | Niosomes | Anticancer | The release of artesunate from the formulations were slow and sustained with enhanced inhibitory effects against MCF-7 and C6 cell lines. | [66] |
| Artesunate | Ethosomes | Antimalarial | Enhanced permeation effect with antimalarial activity against Plasmodium parasite with no sign of re-emergence of malaria infection. | [67] |
| Artemisinin Derivatives | Composition | Drug Delivery System | Application | Advantage | Ref. |
|---|---|---|---|---|---|
| Artemisinin | Multi-walled carbon nanotubes | Carbon-based | Anticancer | Enhanced inhibitory effect on K562 cancer cell lines. | [72] |
| Artemisinin | Multi-walled carbon nanotubes | Carbon-based | Anticancer | Enhanced antitumor effects in tumor-bearing murine model. | [73] |
| Artesunate | Fullerene, hyaluronic acid | Carbon-based | Anticancer | Excellent antitumor activity. | [74] |
| Artemisinin dimer piperazine derivatives | l-α-Phosphatidyl-choline extract from egg | Lipid-based nanoparticles | Anticancer | Enhanced inhibition of the growth of breast cancer cells and induced down-regulation of HER Family Members. | [76] |
| Areether | Soya lecithin, Tween 80 and Pluronic F68 | Lipid-based nanoparticles | Antimalarial | Enhanced bioavailability. | [77] |
| Dihydroxyartemisinin | Miglyol® 812 | Lipid-based nanoparticles | Antimalarial | Enhanced bioavailability. | [78] |
| Artemether | Glyceryl trimyristate (solid lipid) and soybean oil | Lipid-based nanoarticles | Antimalarial | Improved bioavailability and biocompatibility. | [79] |
| Artemisinin | Poly lactic co-glycolic acid | Polymer-based nanoparticles | Antileishmanial | Significant reduction in the parasite load in the liver and spleen than the free artemisinin. | [80] |
| Artemisinin | Poly(ε-capro-lactone) | Polymer-based nanoparticles | Antimalarial | Sustained delivery of artemisinin. | [81] |
| Artesunate | Poly(lactic-co-glycolic)acid- | Polymer-based nanoparticles | Anticancer | Inhibitory effect of the nanoparticles loaded with drug was enhanced on A549, SCC-7, and MCF-7 cancer cell lines. | [82] |
| Artesunate | β-Cyclodextrin, chitosan/lecithin | Polymer-based nanoparticles | Antimalarial | Enhanced drug stability and antimalarial activity. | [83] |
| Dihydroartemisinin | Gelatin and hyaluronan | Polymer-based nanoparticles | Anticancer | Significant inhibition of the proliferation of A549 cells | [84] |
| Artemisinin | Albumin | Polymer-based nanoparticles | Antimalarial | Excellent bioavailability. | [85] |
| Dihydroartemisinin | Poly(lactic-co-glycolic)acid- | Polymer-based nanoparticles | Anticancer | Enhanced anticancer activity. | [86] |
| Artesunate | Fe3O4 | Inorganic-based nanoparticles | Anticancer | Significant cell growth inhibition and apoptosis rate of K562 cell lines. | [87] |
| Artemisinin | Iron nanoparticles | Inorganic-based nanoparticles | Anticancer | Enhanced inhibition of HeLa cell lines. | [88] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aderibigbe, B.A. Design of Drug Delivery Systems Containing Artemisinin and Its Derivatives. Molecules 2017, 22, 323. https://doi.org/10.3390/molecules22020323
Aderibigbe BA. Design of Drug Delivery Systems Containing Artemisinin and Its Derivatives. Molecules. 2017; 22(2):323. https://doi.org/10.3390/molecules22020323
Chicago/Turabian StyleAderibigbe, Blessing Atim. 2017. "Design of Drug Delivery Systems Containing Artemisinin and Its Derivatives" Molecules 22, no. 2: 323. https://doi.org/10.3390/molecules22020323
APA StyleAderibigbe, B. A. (2017). Design of Drug Delivery Systems Containing Artemisinin and Its Derivatives. Molecules, 22(2), 323. https://doi.org/10.3390/molecules22020323