Bovine Colostrum Whey Protein Hydrolysate Inhibits Cell DNA Damage and LDL Oxidation In Vitro
Abstract
:1. Introduction
2. Results
2.1. Degree of Hydrolysis of Whey Protein by Two-Stage Hydrolysis
2.2. Effect of Whey Protein Hydrolysate (WPH) and WPH Fractions on the Fenton Reaction-Induced Oxidative Damage of Deoxyribose
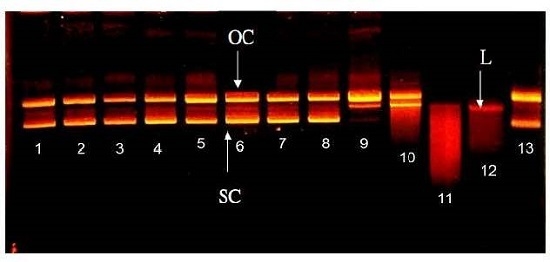
2.3. Effect of WPH and WPH Fractions on Single-Strand DNA Cleavage Induced by Fenton Reaction
2.4. Effect of WPH and WPH Fractions on the Oxidation of 2′-Deoxyguanosine (2′-dG) to 8-OH-2′-dG Induced by Fenton Reaction
2.5. Effect of WPH and WPH Fractions on Bleomycin-Dependent DNA Damage
2.6. The Protective Effects and Inhibition of Oxidative Damages of Biomolecules by WPH and WPH Fractions
2.7. Effects of WPH and WPH Fractions on the Formation of Thiobarbituric Acid Reactive Substances (TBARS) and Conjugated Dienes on LDL Oxidation Induced by Cu2+
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Whey Proteins
4.3. Enzymatic Hydrolysis
4.4. Ultrafiltration (UF)
4.5. Effect of WPH and WPH Fractions on Deoxyribose Damage (Fenton Reaction)
4.6. Effect of WPH and WPH Fractions on DNA Damage (Fenton Reaction)
4.7. Effect of WPH and WPH Fractions on Oxidation of 2′-Deoxyguanosine (Fenton Reaction)
4.8. Effect of WPH and WPH Fractions on Bleomycin-Dependent DNA Damage
4.9. Inhibition of Oxidative Damage of Biomolecules by WPH and WPH Fractions
4.10. LDL Preparation
4.11. LDL Oxidation
4.12. Thiobarbituric Acid Reactive Substances (TBARS)
4.13. Conjugated Diene
4.14. Statistics
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Chen, C.W.; Chiang, S.H.; Wang, S.Y.; Lin, Y.T.; Chang, C.Y. Growth inhibition and differentiating effects of protein hydrolysates from bovine colostrums on human leukemic U937 cell. J. Food Biochem. 2013, 37, 8–17. [Google Scholar] [CrossRef]
- Chen, C.W.; Chiang, S.H.; Chang, C.Y. The inhibition effect of cell DNA oxidative damage and LDL oxidation by bovine colostrums. Molecules 2016, 21, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Palyford, R.D.; Mcdonald, C.E.; Johnson, W.S. Colostrums and milk-derived peptide growth factors for the treatment of gastrointestinal disorders. Am. J. Clin. Nutr. 2000, 72, 5–14. [Google Scholar]
- Ogawa, J.; Sasahara, A.; Yoshida, T.; Sira, M.M.; Futatani, T.; Kanegane, H.; Miyawaki, T. Role of transforming growth factor in breast milk for initiation of IgA production in newborn infants. Early Hum. Dev. 2004, 77, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Palkovicova, L.; Droban, B.; Petrik, J.; Kocan, A.; Trnovec, T.; Picciotto, I.H. Comparison of organochlorine compound concentrations in colostrums and mature milk. Chemosphere 2007, 66, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, K.; Domi, M.; Ando, J. Bovine colostral CD-8 positive cells are potent IFN-γ-producing cells. Vet. Immunol. Immunopathol. 2008, 124, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Paik, H.D.; Yoon, Y.C.; Park, E. Whey protein inhibits iron overload-induced oxidative stress in rats. J. Nutr. Sci. Vitamino 2013, 59, 198–205. [Google Scholar] [CrossRef]
- Chiang, S.H.; Chang, C.Y. Antioxidant properties of casein and whey proteins from bovine colostrum. J. Food Drug Anal. 2005, 13, 57–63. [Google Scholar]
- Wang, X.; Jaio, C.; Wang, T.; Yu, Z. Study on DNA damage induced by the reactive oxygen species generated in situ based on the multi-walled carbon nanotubes and hemoglobin. J. Electroanal. Chem. 2016, 767, 182–187. [Google Scholar] [CrossRef]
- Oliveira, S.C.B.; Oliveira-Brett, A.M. In situ DNA oxidative damage by electrochemically generated hydroxyl free radicals on a boron-doped diamond electrode. Langmuir 2012, 28, 4896–4901. [Google Scholar] [CrossRef] [PubMed]
- Suzuki-Sugihara, N.; Kishimoto, Y.; Saita, E.; Taguchi, C.; Kobayashi, M.; Ichitani, M.; Ukawa, Y.; Sagesaka, Y.M.; Suzuki, E.; Kondo, K. Green tea catechins prevent low-density liporptein oxidation via their accumulation in low-density lipoprotein particles in humans. Nutr. Res. 2016, 36, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D. The LDL, modification hypothesis of atherogenesis: An update. J. Lipid Res. 2009, 50, S376–S381. [Google Scholar] [CrossRef] [PubMed]
- Huxley, P.R.; Neil, H.A. The relation between dietary flavonols and coronary heart disease mortality: A meta-analysis of prospective cohort studied. Eur. J. Clin. Nutr. 2009, 57, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Schildermann, P.A.E.L.; Ten Hoor, F.; Kleinjas, J.C.S. Induction of oxidative DNA damage and early lesions in rat gastro-intestinal epithelium in relation to prostaglandin H synthase-mediated metabolism of butylatedhydroxyanisole. Food Chem. Toxicol. 1995, 33, 99–109. [Google Scholar] [CrossRef]
- Rebeca, B.D.; Pena-Vera, M.T.; Diaz-Castaneda, M. Production of fish protein hydrolysates with bacterial protease, yield and nutritional value. J. Food Sci. 2006, 56, 309–314. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Murcia, A.; Butler, J.; Halliwell, B. Evaluation of antioxidant and prooxidant actions of gallic acid and its derivatives. J. Agric. Food Chem. 1993, 41, 1880–1885. [Google Scholar] [CrossRef]
- Hsieh, C.H.; Yen, G.C. Antioxidant actions of Du-Zhong (Eucommia ulmoides oliv.) toward oxidative damage in biomolecules. Life Sci. 2000, 66, 1387–1400. [Google Scholar] [CrossRef]
- Kobayashi, S.; Uead, K.; Komano, T. The effects of metal ions on the DNA damage induced by hydrogen peroxide. Agric. Biol. Chem. 1990, 54, 69–76. [Google Scholar] [PubMed]
- Smith, C.; Halliwell, B.; Aruoma, O.I. Protection by albumin against the pro-oxidant actions of phenolic dietary components. Food Chem. Toxicol. 1992, 30, 483–489. [Google Scholar] [CrossRef]
- Treml, J.; Šmejkal, K. Flavonoids as potent scavengers of hydroxyl radicals. Compr. Rev. Food Sci. Food Saf. 2016, 15, 720–738. [Google Scholar] [CrossRef]
- Bayram, T.; Pekmze, M.; Arda, N.; Yalcin, A.S. Antioxidant activity of whey protein fractions isolated by gel exclusion chromatography and protease treatment. Talanta 2008, 75, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Brandelli, A.; Daroit, D.J.; Correa, A.P.F. Whey as a source of peptides with remarkable biological activities. Food Res. Int. 2015, 73, 149–161. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Halliwell, B.; Williamson, G. In vitro methods for characterizing potential prooxidant and antioxidant actions of nonnutritive substances in plant foods. In Antioxidant Methodology In Vivo and In Vitro Concepts; Aruoma, O.I., Cuppett, S.L., Eds.; AOCS Press: Champaign, IL, USA, 1998; Volume 10, pp. 173–204. [Google Scholar]
- Erdem, G.; Öner, C.; Önal, A.M.; Kisakürek, D.; Öhüs, A. Free radical mediated interaction of ascorbic acid and ascorbate/Cu(II) with viral and plasmid DNAs. J. Biosci. 1994, 19, 9–17. [Google Scholar] [CrossRef]
- Stadtman, E.R. Ascorbic acid and oxidative inactivation of protein. Am. J. Clin. Nutr. 1991, 54, 1125–1128. [Google Scholar]
- Hu, M.L.; Shih, M.K. Ascorbic acid inbibits lipid peroxidation but enhances DNA damage in rat liver nuclei incubated with iron ions. Free Radic. Res. 1997, 26, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Takagi, A.; Sai, K.; Umemura, T.; Hasegawa, R.; Kurokawa, Y. Inhibitory effects of vitamin E and ellagic acid on 8-hydroxydeoxyguanosine formation in liver nuclear DNA of rats treated with 2-nitropropane. Cancer Lett. 1995, 91, 139–144. [Google Scholar] [CrossRef]
- Kasai, H.; Nishimura, S. Hydroxylation of deoxyguanosine at the C-8 position by ascorbic acid and other reducing agents. Nucleic Acids Res. 1984, 12, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Shibutani, S.; Takeshita, M.; Grollman, A.P. Insertion of specific bases during DNA synthesis past the oxidation-damage base 8-oxo-dG. Nature 1991, 349, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Shigenaga, M.K.; Ames, B. Assay for 8-hydroxy-20-deoxyguanosine: A biomarker of in vivo oxidative DNA damage. Free Radic. Biol. Med. 1991, 10, 211–216. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y.; Peng, H.H. Antioxidant and pro-oxidant effects of various tea extracts. J. Agric. Food Chem. 1997, 45, 30–34. [Google Scholar] [CrossRef]
- Povirk, L.F.; Austin, M.J.F. Genotoxicity of bleomycin. Mutat. Res. 1991, 257, 127–143. [Google Scholar] [CrossRef]
- Huet, J.; Laval, F. Potentiation of cell killing by inhibitors of poly(adenosinediphosphate-ribose) synthesis in bleomycin-treated Chineses hamster ovary cells. Cancer Res. 1985, 45, 987–991. [Google Scholar] [PubMed]
- Zhang, P.; Omaye, S.T. β-Arotene: Interactions with α-tocopherol and ascorbic acid in microsomal lipid peroxidation. J. Nutr. Biochem. 2001, 12, 38–45. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis, 18th ed.; Revised; Association of Official Analytical Chemists: Washington, DC, USA, 2006. [Google Scholar]
- Moran, J.F.; Klucas, R.V.; Grayer, R.J.; Abian, J.; Becana, M. Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: Prooxidant and antioxidant properties. Free Radic. Biol. Med. 1997, 22, 861–870. [Google Scholar] [CrossRef]
- Yagi, K. A simple fluorometric assay for lipid peroxides in blood serum or plasma. In CRC Hand Book of Free Radicals and Antioxidants in Biomedicine; Miquel, J., Quintanilha, A.T., Weber, H., Eds.; CRC Press: Boca Raton, FL, USA, 1989; Volume 3, pp. 215–218. [Google Scholar]
- Agin, D.; Gallagher, D.; Wang, J. Effects of whey protein and resistance exercise on body cell mass, muscle strength, and quality of life in women with HIV. AIDS 2001, 7, 431–440. [Google Scholar] [CrossRef]
- Bounous, G. Whey protein concentrate (WPC) and glutathione modulation in cancer treatment. Anticancer Res. 2000, 20, 4785–4792. [Google Scholar] [PubMed]
- Watanabe, A.; Okada, K.; Shimizu, Y. Nutritional therapy of chronic hepatitis by whey protein (non-heated). J. Med. 2000, 31, 283–302. [Google Scholar] [PubMed]
- Lands, L.C.; Grey, V.L.; Smountas, A.A. Effect of supplementation with a cysteine donor on muscular performance. J. Appl. Physiol. 1999, 87, 1381–1385. [Google Scholar] [PubMed]
- Sample Availability: Samples of the compounds WHP and WHP fractions are available from the authors.






| Addition to RM * | 8-OH-2′-dG (μg/mL) | ||
|---|---|---|---|
| WPH | WPH Fraction (>10 kDa) | WPH Fraction (<10 kDa) | |
| Blank (PBS) | 0.47 ± 0.05 b,** | 0.47 ± 0.05 b,** | 0.47 ± 0.05 b,** |
| 15 mM ascorbic acid | 10.24 ± 0.19 a,A | 10.24 ± 0.19 a,A | 10.24 ± 0.19 a,A |
| 1 mg/mL | 0.25 ± 0.04 c,B | 0.06 ± 0.01 c,A | 0.09 ± 0.01 c,A |
| 2 mg/mL | 0.12 ± 0.02 d,B | 0.04 ± 0.0 c,A | 0.06 ± 0.01 c,A |
| 4 mg/mL | 0 | 0 | 0.03 ± 0.0 c |
| 6 mg/mL | 0 | 0 | 0 |
| 8 mg/mL | 0 | 0 | 0 |
| 10 mg/mL | 0 | 0 | 0 |
| Addition to RM* | Protective Effect of 2′-dG | Bleomycin–Fe3+/Asc | Fe2+-EDTA/H2O2/Asc. | |||
|---|---|---|---|---|---|---|
| 8-OH-2′-dG (μg/mL) | Inhibition (%) | Absorbance at 532 nm | Inhibition (%) | 8-OH-2′-dG (μg/mL) | Inhibition (%) | |
| Ascorbic acid | 5.91 ± 0.28 a,** | 0.183 ± 0.01 a,** | 10.11 ± 0.51 a,** | |||
| WPH | 1.77 ± 0.18 b | 70.05 ± 4.38 b | 0.172 ± 0.02 b | 6.01 ± 0.15 b | 7.21 ± 0.45 b | 28.68 ± 1.61 a |
| WPH fraction (>10 kDa) | 1.25 ± 0.19 c | 78.85 ± 4.09 a | 0.168 ± 0.0 b | 8.20 ± 0.62 a | 7.03 ± 0.45 b | 30.46 ± 1.90 a |
| WPH fraction (<10 kDa) | 1.81 ± 0.10 b | 69.37 ± 1.30 b | 0.172 ± 0.01 b | 6.01 ± 0.49 b | 7.00 ± 0.55 b | 30.76 ± 2.17 a |
| Concentration (mg/mL) | WPH | WPH Fraction (>10 kDa) | WPH Fraction (<10 kDa) | Concentration (mg/mL) | WPH | WPH Fraction (>10 kDa) | WPH Fraction (<10 kDa) |
|---|---|---|---|---|---|---|---|
| TBARS (n mol/mL) | Lag Time * (min) | ||||||
| Blank | 5.13 ± 0.01 a,** | 5.13 ± 0.01 a,** | 5.13 ± 0.01 a,** | Blank | 90 | 90 | 90 |
| 0.001 | 4.98 ± 0.02 a | 4.65 ± 0.02 a,b | 4.10 ± 0.05 b | 0.1 | 180 | 180 | 180 |
| 0.01 | 4.82 ± 0.01 a | 4.22 ± 0.04 b | 4.22 ± 0.04 b | 1.0 | 210 | 210 | 210 |
| 0.1 | 4.19 ± 0.03 b | 3.89 ± 0.01 b,c | 4.85 ± 0.07 a | 10 | 240 | 270 | 270 |
| 1.0 | 3.70 ± 0.01 c | 3.65 ± 0.01 c | 4.84 ± 0.02 a | - | - | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, S.-H.; Wang, S.-Y.; Chang, C.-Y.; Chen, C.-W. Bovine Colostrum Whey Protein Hydrolysate Inhibits Cell DNA Damage and LDL Oxidation In Vitro. Molecules 2017, 22, 456. https://doi.org/10.3390/molecules22030456
Chiang S-H, Wang S-Y, Chang C-Y, Chen C-W. Bovine Colostrum Whey Protein Hydrolysate Inhibits Cell DNA Damage and LDL Oxidation In Vitro. Molecules. 2017; 22(3):456. https://doi.org/10.3390/molecules22030456
Chicago/Turabian StyleChiang, Shu-Hua, Shiu-Yu Wang, Chi-Yue Chang, and Chih-Wei Chen. 2017. "Bovine Colostrum Whey Protein Hydrolysate Inhibits Cell DNA Damage and LDL Oxidation In Vitro" Molecules 22, no. 3: 456. https://doi.org/10.3390/molecules22030456
APA StyleChiang, S. -H., Wang, S. -Y., Chang, C. -Y., & Chen, C. -W. (2017). Bovine Colostrum Whey Protein Hydrolysate Inhibits Cell DNA Damage and LDL Oxidation In Vitro. Molecules, 22(3), 456. https://doi.org/10.3390/molecules22030456